Insights into Treated Articles and Biocidal Products: Understanding Differences & Regulations
Explore the concept of treated articles, their success strategies, and the distinctions from biocidal products. Learn about examples, obligations, and authorization requirements for treated articles in the EU market.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Treated Articles success strategies for treated articles and differences between treated articles and biocidal products Yana Trubitsyna Belgrade, May 2023
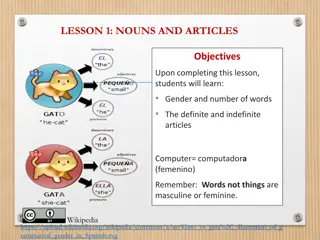
What is a treated article? Mixture, substance or article treated with biocidal products The biocidal products protect the articles from harmful organisms like pests, mould and bacteria Function + additional advantage given by biocidal product treatment
Examples of treated articles Examples of treated articles: A paint that contains an in-can preservative (mixture) Socks containing silver fibre to prevent odour (article incorporating a biocide) A refrigerator that has been treated with substances to prevent mould and odour (article).
Treated Article X Biocidal Product A treated article that has a primary biocidal function shall be considered a biocidal product. Example: An anti-bacterial wipe is a biocidal product not a treated article because its sole purpose is to control bacteria.
Treated articles - obligations Article 3 of BPR Making available on the market making available on the market means any supply of a biocidal product or of a treated article for distribution or use in the course of a commercial activity, whether in return for payment or free of charge; Placing on the market placing on the market means the first making available on the market of a biocidal product or of a treated article;
Treated articles - obligations Authorization of treated article Articles that have been treated with a biocide do not need authorisation, but they can only be placed on the EU market when the active substance in the biocide has been approved for the specific use (Article 58 of BPR) Treated article should not be placed on the market, unless all active substances are approved for the specific use.
Treated articles - obligations Labelling The label should contain: A statement that the treated article incorporates biocidal products; Where substantiated, the biocidal property attributed to the treated article; Name(s) of all active substances contained in the biocidal products; Name(s) of all nanomaterials contained in the biocidal products, followed by the word nano in brackets; Any relevant instructions for use, including any precautions to be taken, if this is necessary to protect humans, animals and the environment.
Treated articles - obligations Labelling Other requirements to the label: Cleary visible Easily readable Appropriately durable Could be printed on the packaging (because of size or function) Official language of Member state, unless MS provides otherwise.
Treated articles - obligations Obligation to provide information to the consumer Upon the request of the consumer, the supplier of a treated article, shall provide the information on the biocidal treatment of the treated article.
Transitional measures
Transitional measures Since 1 March 2017, companies are no longer allowed to place on the EU market any articles treated with (or intentionally incorporating) a biocidal product with an active substance which is not already approved, under review or listed in Annex I to the BPR. The possibility to place treated article on the market is defined by the status of the active substance. There are 4 scenarios when it is possible to place the treated article on the market.
Transitional measures 1. Active substance has been assessed and approved for the biocide used to treat the article. 2. The active substance is under assessment in the Review Programme, but the decision is not yet published. 3. The active substance is not in the Review Programme, but an application for approval of the active substance was submitted before 1 September 2016 4. The active substance is included in Annex I to the BPR the list of substances that do not give rise to concern.
Transitional measures If an active substance is not approved or the application is rejected (for example, due to failure to pay the relevant fee), articles treated with or incorporating a biocidal product with that active substance should no longer be placed on the market after 180 days from the decision.