Introduction to Carbonyl Compounds
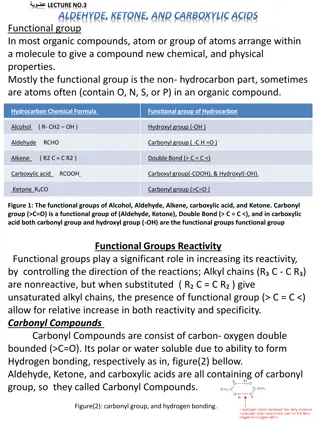
Carbonyl compounds, such as aldehydes and ketones, contain the C=O group known as the carbonyl group. Aldehydes have the group linked to hydrogen, while ketones have it linked to alkyl groups. These compounds can be symmetrical or unsymmetrical depending on the alkyl groups attached to the carbonyl carbon. Nomenclature systems include Common and IUPAC systems for naming these compounds, with aromatic aldehydes being of two types. Ketones are named using common or IUPAC systems as well.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
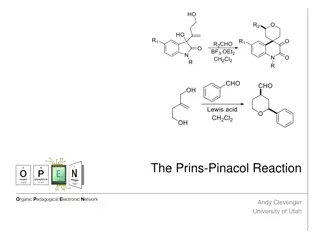
Aldehydesandketonescontains-C=Ogroup. ThisC=O groupisknownasCarbonylgroup hencethesecompoundsarecalledasCarbonyl compounds.Ifoneofthevalencyofthe Carbonylgroupislinkedtohydrogenthat compoundisknownasaldehyde.
Ifthetwovalanciesof theCarbonylcarbonis linkedtoalkylgroupsthosecompoundsare knownasketones.If thetwoalkylgroups attachedtotheCarbonylcarbonissamethey arecalledsymmetricalketones.If thetwoalkyl groupsaredifferenttheyarecalledas unsymmetricalketones.
Symmetrical Unsymmetrical
Nomenclature:Fornamingcarbonyl compoundstwosystemsareused(1) Common system,(2)IUPACSystem
Commonsystem: Inthissystemaldehydesare namedafterthecarboxylicacidswhichthey formonoxidation.Thesuffixofthecommon namesofacidsis-icacid.Thissuffixisdeleted andthewordaldehydeisadded.Forexample CH3CHOonoxidationgivesaceticacid. ThereforeCH3CHOiscalledacetaldehyde.
IUPACSystem:Accordingtothissystem namesofaldehydesareobtainedfromthe namesof thecorrespondinghydrocarbons.In thenameofthehydrocarbonletter e is replacedby al .
Aromaticaldehydesareoftwodifferenttypes :(i)Compoundsinwhichaldehydesgroup ( CHO)isdirectlyattachedaromatic nucleus.Forexample
(ii)Compoundsinwhichaldehydegroupis presentinthesidechain.
Ketones:Fornamingketonetwosystems are adopted. (1)Commonname:Inthissystemthenames areobtainedbyaddingketonetothenamesof thealkylgroupsattachedtotheCarbonyl group.
(2)IUPACsystem:(a)Numberingisgivento thecarbonchainuchthattheCarbonylgroup getslowestnumber.(b) Inthenameof the correspondingalkaneletter e isreplacedby 'one togetthenameofthecompound (ketone).
Aromaticketones:Inaliphaticketonesifone ortwohydrocarbongroupsattachedto Carbonylgrouparereplacedbyarylgroups theyarecalledaromatic ketones.Theyare designatedasalkarylordiarylketones.
KetonescontainingtheCarbonylgroup attached tobenzeneringarenamedphenones.
Aldehydesshowchainandfunctional isomerism.Ketonesshowchain,functional andmetamerism.
Keto-Enoltautomerism: Tautomerism may be defined as a phenomenon in which a single compound exists in two readily interconvertible structures that differ markedly in the relative position of at least one atomic nucleus, generally hydrogen. The two different structures are knownastautomersofeachother.
Tautomerismiscausedbythemigrationofan atom,generallyhydrogenfromonepositionto anotherinthesamemolecule.Iftheatom migratesfromitsposition(1)totheadjacent position(2),thetautomerismissaidtobeof dyadtype,whileitisof triadtypewhenan atommigratesfromatomItoatom3.
Noteherethehydrogenmigratesfromatom1 (C) to3(O).Triadtypeoftautomerism ismore common,againinthistype,keto-enol tautomerismisthemostimportant.Inthis type,oneform(tautomer)existsasaketone whiletheotherexistsasanenol.Thetwo simplestexamplesareofacetoneandphenol.
Keto-enol tautomerism in acetoacetic ester is proved by thefactthatunderordinaryconditionsthecompound gives the properties of the ketonic group (reaction with hydroxylamine to form oxime, reaction with phenylhydrazine to form phenylhydrazone, reduction to form secondary alcohol etc.) as well as that of the enolic group (reaction with PCl5, NH3, bromine water, FeCl3, acidiccharacter etc.)
Note that in all the examples of keto-enol tautomerism the two isomeric forms are interconvertible by the migration of a proton from one atom (carbon) to other with the simultaneous shifting of bonds. Remember that keto-enoltautomerismispossibleonlyinthosealdehydes andketoneswhichhaveatleastonealpha hydrogen atomwhichcanconvert theketonicgrouptotheenolic group.Examinethefollowingcompounds.