Ionic Compound Nomenclature Challenge - Polyatomic Ion Quiz
Today's objective is to understand and practice naming ionic compounds, focusing on Type I and Type II ionic compounds. The agenda includes a Polyatomic Ion Quiz 3 and writing ionic formulas. The homework involves reviewing various ionic compounds and their nomenclatures. The content also covers three types of ionic compounds: Type 1 - Binary compounds, Type 2 - Binary compounds with variable charge cations, and Type 3 - Polyatomic ion compounds. You will work on naming and writing formulas for different compounds, focusing on metals, nonmetals, and transition metals.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Chemistry Jan 23, 2018 P3 Challenge- Polyatomic Ion Quiz 3 only best attempt counts (Caught cheating on any trial 0 averaged with best score on all others) Get out Ionic Compounds Homework Table HMK when done. Today s Objective Ionic Compound Nomenclature (You will need your colored periodic table today)
Chemistry Jan 23, 2018 Objective Ionic compound nomenclature Agenda Polyatomic ion quiz 3 Writing ionic formulas HMK Type I Ionic Nomenclature Type II Ionic Nomenclature Polyatomic ions Assignment: Ionic Compounds Worksheet
Homework Review zinc iron (II) iron (III) gallium silver lead (IV) chloride ZnCl2 FeCl2 FeCl3 GaCl3 AgCl PbCl4 acetate Zn(C2H3O2)2 Fe(C2H3O2)2 Fe(C2H3O2)3 Ga(C2H3O2)3 AgC2H3O2 Pb(C2H3O2)4 nitrate Zn(NO3)2 Fe(NO3)2 Fe(NO3)3 Ga(NO3)3 AgNO3 Pb(NO3)4 oxide ZnO FeO Fe2O3 Ga2O3 Ag2O PbO2 nitride Zn3N2 Fe3N2 FeN GaN Ag3N Pb3N4 sulfate ZnSO4 FeSO4 Fe2(SO4)3 Ga2(SO4)3 Ag2SO4 Pb(SO4)2
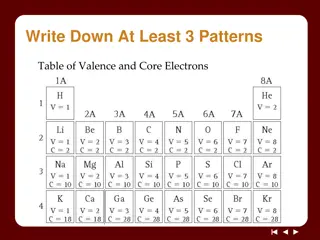
Three types of ionic compounds Type 1 - Binary compound (contains only two elements) and cation charge is known because atom can form only one type of ion. Metal forms only one type of ion: Groups 1A, 2A, Al3+, Ga3+, Zn2+, Cd2+, and Ag+ Type 2 Binary compound with variable charge cations. Transition metals and representative metals. Type 3 Polyatomic ion compound contains three or more elements contained within one or two polyatomic ions.
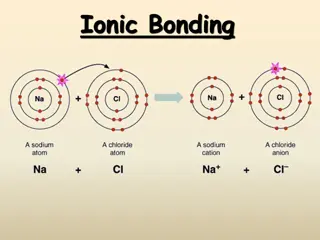
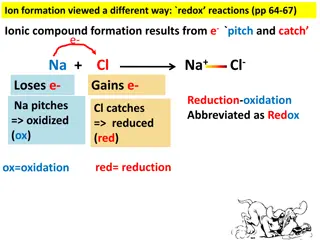
Type 1 Ionic Compounds Formed by electron transfer from a metal to a nonmetal Metal forms only one type of ion: Groups 1A, 2A, Al3+, Ga3+, Zn2+, Cd2+, and Ag+ Name as metal nonmetalide Name sodium chloride calcium oxide lithium fluoride beryllium nitride Formula Formula KBr Al2O3 ZnI2 Ag2S Name
Type 2 Ionic compounds Transition Metals Most transition metals and group 13-16 metals form multiple ions. Cr, Fe and Co form 2+ and 3+ ions Cu forms 1+ and 2+ ions Sn, Pb form 2+ and 4+ ions Mercury (II) is Hg2+, while Mercury (I) is Hg22+ Named as metal (roman numeral for charge on metal) nonmetalide Name lead (II) oxide tin (IV) fluride copper (I) bromide iron (III) selenide Formula Formula CuO Au2S CoP Cr3N2 Name
Type 3 Ionic compounds polyatomic ions Formula will have more than two elements Often includes oxygen Often includes () around a polyatomic ion Name as cation anion Name sodium carbonate tin (II) cyanide ammonium nitrate barium sulfate Formula Formula CuOH Mg(ClO2)2 FePO4 LiN02 Name
Exit Slip - Homework Exit Slip: Name Ca(OH)2 Write the formula for Iron (II) sulfate What s Due? (Pending assignments to complete.) Ionic Compounds Worksheet What s Next? (How to prepare for the next day) Read Holt p159-175