Kinetic Theory of Gases and Effusion Studies
Explore the key ideas in kinetic theory, including pressure calculation, internal energy density, velocity distributions, and effusion processes in classical ideal gases. Understand the validity of assumptions made and the comparison of particle speeds inside and outside a container.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
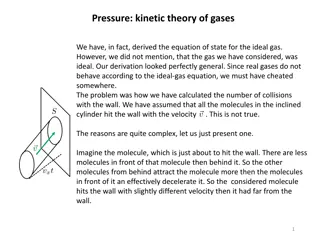
KT2: key ideas Kinetic calculation of pressure; relation to internal energy density Spherical velocity-space coordinates and the distribution of particle speeds The equilibrium pdf for a homogeneous, isotropic (ideal) gas is Maxwell s distribution Kinetic theory definition of temperature (spread in velocities about mean)
KT3: key ideas Classical ideal gas assumptions and validity Effusion = gas leak through tiny hole Kinetic derivation of effusion rate Useful for measuring particle flux in applications Velocity distribution for effusing beam Allows for non-perturbative measurement of velocity distribution inside container Simple kinetic theory expression for collisional mean-free-path and collision time
KT3: key ideas Classical ideal gas assumptions and validity Effusion = gas leak through tiny hole Kinetic derivation of effusion rate Useful for measuring particle flux in applications Velocity distribution for effusing beam Allows for non-perturbative measurement of velocity distribution inside container Simple kinetic theory expression for collisional mean-free-path and collision time
Effusion Wall area = A Hole diameter = d detector z
Will the average speed of the particles that escape the container be greater than, less than, or equal to the average speed of the particles in the container?
Effusion Wall area = A Hole diameter = d detector z
KT3: key ideas Classical ideal gas assumptions and validity Effusion = gas leak through tiny hole Kinetic derivation of effusion rate Useful for measuring particle flux in applications Velocity distribution for effusing beam Allows for non-perturbative measurement of velocity distribution inside container Simple kinetic theory expression for collisional mean-free-path and collision time