LDV/SOF Japanese Study for Genotype 1 Chronic HCV Infection
Explore the LDV/SOF Japanese Study (GS-US-337-0113) focusing on the treatment of genotype 1 Chronic HCV infection in Japanese patients. The study includes baseline characteristics, treatment regimens, SVR12 rates, and patient outcomes. Findings indicate high SVR rates with LDV/SOF and LDV/SOF + RBV treatments. The study also covers patients with NS5A RAV at baseline, adherence rates, and virological failure cases.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
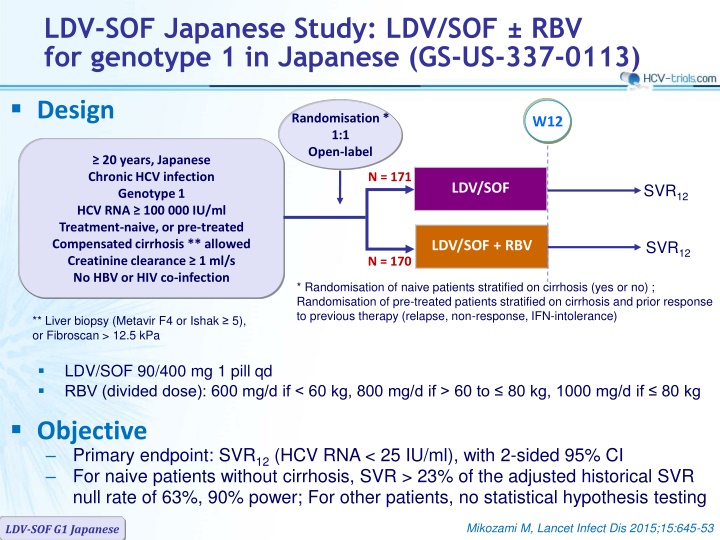
LDV-SOF Japanese Study: LDV/SOF RBV for genotype 1 in Japanese (GS-US-337-0113) Design Randomisation * 1:1 Open-label W12 20 years, Japanese Chronic HCV infection Genotype 1 HCV RNA 100 000 IU/ml Treatment-naive, or pre-treated Compensated cirrhosis ** allowed Creatinine clearance 1 ml/s No HBV or HIV co-infection N = 171 LDV/SOF SVR12 SVR12 LDV/SOF + RBV N = 170 * Randomisation of naive patients stratified on cirrhosis (yes or no) ; Randomisation of pre-treated patients stratified on cirrhosis and prior response to previous therapy (relapse, non-response, IFN-intolerance) ** Liver biopsy (Metavir F4 or Ishak 5), or Fibroscan > 12.5 kPa LDV/SOF 90/400 mg 1 pill qd RBV (divided dose): 600 mg/d if < 60 kg, 800 mg/d if > 60 to 80 kg, 1000 mg/d if 80 kg Objective Primary endpoint: SVR12(HCV RNA < 25 IU/ml), with 2-sided 95% CI For naive patients without cirrhosis, SVR > 23% of the adjusted historical SVR null rate of 63%, 90% power; For other patients, no statistical hypothesis testing Mikozami M, Lancet Infect Dis 2015;15:645-53 LDV-SOF G1 Japanese
LDV-SOF Japanese Study: LDV/SOF RBV for genotype 1 in Japanese (GS-US-337-0113) Baseline characteristics, and disposition LDV/SOF, N = 171 LDV/SOF + RBV, N = 170 Mean age, years Female, % Genotype 1a / 1b, % IL28B CC genotype, % HCV RNA log10IU/ml, mean Cirrhosis, % Treatment history, % Naive Pre-treated PEG-IFN + RBV PI + PEG-IFN + RBV Other Response to previous treatment Non-response Breakthrough or relapse IFN-intolerance Discontinuation, N 60 60 59 57 4 / 96 50 6.6 24 2 / 98 46 6.6 21 49 51 61 19 19 49 51 54 26 20 33 50 17 0 32 51 17 2 (1 AE, 1 death) Mikozami M, Lancet Infect Dis 2015;15:645-53 LDV-SOF G1 Japanese
LDV-SOF Japanese Study: LDV/SOF RBV for genotype 1 in Japanese (GS-US-337-0113) SVR12(HCV RNA < 25 IU/ml), % (95% CI), ITT LDV/SOF LDV/SOF + RBV 100 100 100 100 100 97.2 98 96.4 % (94.9-100) (95.7-100) (98-100) (95.8-100) (95.9-100) (90.2-99.8) (95-100) (89.8-99.2) 100 80 60 40 20 N = 171 170 83 83 88 87 70 71 0 All All Naive Experienced Naive, no cirrhosis * * p < 0.0001 vs SVR null rate of 63%, for both comparisons Mikozami M, Lancet Infect Dis 2015;15:645-53 LDV-SOF G1 Japanese
LDV-SOF Japanese Study: LDV/SOF RBV for genotype 1 in Japanese (GS-US-337-0113) Patients with NS5A RAV at baseline, N = 76 SVR12in 42/42 on LDV/SOF SVR12in 33/34 on LDV/SOF + RBV 1 virological failure Treatment-naive, genotype 1b, 55-year-old woman without cirrhosis who was receiving LDV/DOF + RBV, relapse by post-treatment W4 after completion of treatment. Adherence rates > 99% for both LDV/SOF and RBV (800 mg daily) Baseline NS5A RAV: Y93H (> 99%) NS5A. No other NS5A RAVs were detected at post-treatment W4 No NS5B RAVs and no treatment-emergent variants were detected in any patient at any timepoint tested Mikozami M, Lancet Infect Dis 2015;15:645-53 LDV-SOF G1 Japanese
LDV-SOF Japanese Study: LDV/SOF RBV for genotype 1 in Japanese (GS-US-337-0113) Adverse events LDV/SOF N = 171 0 0 3 0 0 1 1 1 LDV/SOF + RBV N = 170 2 * 1 2 1 ** 1 ** 0 0 0 Treatment discontinuation due to adverse event, N Death Serious adverse events, N Acute myocardial Infarction Cardiac arrest Hepatocellular carcinoma Oesophageal varices haemorrhage Wrist fracture Common adverse events, % Nasopharyngitis Anemia Headache Pruritus Rash Malaise Stomatitis Nausea Decreased hemoglobin : < 10 g/dl / < 8.5 g/dl, N Lymphocyte count 350-500/mm3, N Neutrophil count 500-750/mm3, N Platelet count 25 000-50 000/mm3, N * drug eruption, N = 1, morbilliform rash, N = 1 ; ** related to study drug 29 2 7 4 3 5 4 3 4 / 1 3 2 1 24 14 9 8 8 5 6 5 10 / 0 1 0 0 Mikozami M, Lancet Infect Dis 2015;15:645-53 LDV-SOF G1 Japanese
LDV-SOF Japanese Study: LDV/SOF RBV for genotype 1 in Japanese (GS-US-337-0113) Summary In this trial, 12 weeks of treatment with the fixed-dose combination of LDV/SOF without RBV was well tolerated and resulted in SVR12 in all 171 patients (100%) treated, including patients typically difficult to treat, including those with cirrhosis, or baseline NS5A RAVs, and those who had previously not responded well to other HCV treatment regimens, including PI-based therapies The addition of RBV to LDV/SOF let to a SVR12of 97%, and was associated with an increased number of patients who had adverse events Limitations of the study Open-label design Absence of an active comparator Mikozami M, Lancet Infect Dis 2015;15:645-53 LDV-SOF G1 Japanese






















