Lewis Structures and Chemical Formulas
Learn about drawing Lewis structures, different types of chemical formulas (molecular, empirical, structural), and how to draw Lewis structures step by step. Explore the concept of valence electrons and octet electrons in molecules.
Uploaded on | 1 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Drawing Lewis Structures They re little pictures of molecules! This is Gilbert Lewis, for whom Lewis structures are named. He won the World Cheesecake Eating Championship Contest each year from 1927-1952, despite having died in March, 1946.
There are three types of chemical formula: There are three types of chemical formula: 1. Molecular formulas: They tell you exactly how many atoms of every element are present. This is the type you normally think of when you think formula. Example: C2H4 has two atoms of carbon and four atoms of hydrogen 2. Empirical formulas: These are reduced formulas that tell you the ratio of the atoms of each element to each other. Example: The empirical formula of C2H4 is CH2 (both subscripts divided by 2) These really aren t used much anymore, though there are types of chemical analysis that still use them (which is why you need to know them).
3. Structural formulas 3. Structural formulas Structural formulas show where every atom in the molecule are. Examples: Newman projection (organic chemistry) Fischer projection (biochemistry) Lewis structure (widely used throughout all branches of chemistry
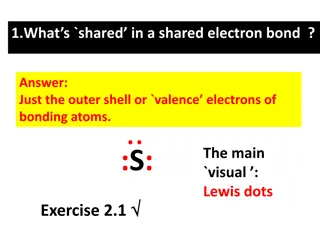
How To Draw Lewis Structures: Example CH How To Draw Lewis Structures: Example CH4 4 1. Count the total number of valence electrons in the molecule This is done by finding the total number of valence electrons that each element has, multiplied by the number of atoms of this element. In our example (CH4): C: 4 valence electrons x 1 atom of C = 4 valence electrons H: 1 valence electron x 4 atoms of H = 4 valence electrons Total: 8 valence electrons
1. Valence electrons (continued) 1. Valence electrons (continued) If you re trying to find the number of valence electrons for a polyatomic ion, adjust your count: If it s a cation, subtract the amount of positive charge from the count. If it s an anion, add the amount of negative charge to the count. Example: OH-1 will have a total of 6 valence electrons Four from carbon One from hydrogen One from the -1 charge
2. Find the number of octet electrons for the 2. Find the number of octet electrons for the molecule molecule The rules for doing this: Hydrogen ALWAYS wants 2 octet electrons! Beryllium ALWAYS wants 4 octet electrons! Boron wants 6 octet electrons for neutral molecules, 8 in anions ALL OTHER ELEMENTS ALWAYS WANT 8 OCTET ELECTRONS! In our example (CH4): C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 atoms = 8 octet electrons Total: 16 octet electrons
3. Find the number of bonding electrons in 3. Find the number of bonding electrons in the molecule the molecule These are the electrons that form the covalent bonds in the molecule. How to do this: Subtract the valence electrons from the octet electrons (or, if you ve forgotten which is which, the small number from the big one) In our example (CH4): 16 octet electrons 8 valence electrons = 8 bonding electrons
4. Find the number of covalent bonds 4. Find the number of covalent bonds If you have the number of bonding electrons, and there are two electrons in each bond, then you find the number of covalent bonds by dividing the bonding electrons by 2. In our example (CH4): 8 bonding electrons divided by 2 = 4 covalent bonds
5. Draw your molecule 5. Draw your molecule Here s where you just go ahead and draw the molecule for which you re trying to find the Lewis structure. There are rules for doing this, but they won t all fit on this slide. As a result, I m going to put them on the next slide. So get ready for that.
5. Drawing the Lewis Structure (continued) 5. Drawing the Lewis Structure (continued) The rules you must follow: Hydrogen and the halogens ALWAYS bond once! Oxygen s family and beryllium bond twice in neutral compounds and one or two times in ions. Nitrogen s family and boron bond three times in neutral compounds and two, three, or four times in ions. Carbon s family ALWAYS bonds four times! Any arrangement of atoms that you put together that has the needed number of bonds you saw in step 4 and the number of bonds you see above for each atom is completely valid!
In our example: Let s go over what we know for sure: We have one carbon atom and four hydrogen atoms The final compound will have four covalent bonds Carbon will have four bonds and hydrogen will have one bond. ANY structure that follows all of these guidelines is completely valid.
So, where do I get started with my drawing? So, where do I get started with my drawing? Typically, a good way to start is to draw the atom that can form the highest number of bonds in the middle and arrange the other atoms around it. This may not always work, but it works more often than not. (Helpful advice: If you really have no idea where to start, just start putting stuff down at random. It s a good way to get moving!)
For some compounds, youll be finished at this For some compounds, you ll be finished at this point. However, for others, you ll have to keep point. However, for others, you ll have to keep going. As a result, let s look at steps 6 and 7 going. As a result, let s look at steps 6 and 7
6. When youre done drawing your structure, add 6. When you re done drawing your structure, add pairs of electrons until all atoms have the same pairs of electrons until all atoms have the same number of octet electrons around them that they number of octet electrons around them that they want (from step 2) want (from step 2) Our example: NH3. Go through steps 1-5 and come up with the basic structure, then we ll meet back here. This is what bottles of ammonia look like in Russia, in case you were wondering.
Well do this on the board, because it will make more sense that way. Again, step 6 says that when you re drawing your structure, add pairs of electrons until all atoms have the same number of octet electrons they want (from step 2). A reminder of how many this is: Hydrogen wants 2 Beryllium wants 4 Boron wants 6 in neutral compounds and 8 in polyatomic ions Everything else wants 8 electrons
Step 7: Add charges to atoms if possible Step 7: Add charges to atoms if possible How to do this: Compare the number of electrons that an atom OWNS (i.e. one per bond, two per lone pair) with the number of valence electrons it normally has (from the periodic table). directly attached to it to the number of octet electrons it wants. If you look at the formula for NH3, there s no charge shown. As a result, you don t even need to do this step. Since OH- has a negative charge, we need to do the math to figure out what atom it s stuck to. (Let s do that now)
Lets do some more examples: CO2 C2H4 SiF4 NO3-1 I ll probably make some of you put these on the board or something. It s not really a very handy thing for PowerPoint.
Uh oh NO3-caused some problems, didnt it? We can draw it in one of three ways, all of which are correct:
So, which one is So, which one is actually actually correct? correct? All of them. Sort of. These structures are resonance structures structures which have the same relative arrangement of atoms but different positions of the electrons/bonds/charges. All of them are sort of correct. And none of them is completely correct.
The actual structure is an average of the The actual structure is an average of the resonance structures resonance structures In cases like this, the electrons are actually delocalized. For the nitrate ion, each of the N-O bonds isn t either singly or double-bonded, but all have equivalent 1 2/3 bonds. If we were to draw what it actually looks like, this is the best we can do: Because this structure is hard to visualize, we typically draw each of the resonance structures with double-headed arrows between them to show that they re equivalent. Let s see on the next slide
The correct way to draw resonance structures The correct way to draw resonance structures
And now, it is time to practice Worksheet is forthcoming.