LINE-1 Transcription in Dogs Using RNA Sequencing
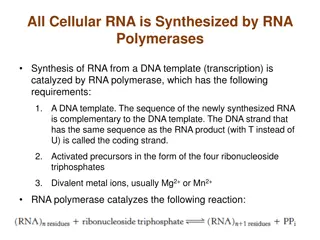
The project explores LINE-1 transcription in dogs using RNA-sequencing data. LINE-1 is a retrotransposon present in a significant portion of the human and dog genomes and is associated with various diseases. The goals involve locating LINE-1s in the genome, identifying highly active LINE-1s, and confirming findings using RT-PCR clones and long-read RNA-sequencing. Techniques such as RepeatMasker and ORFik are employed to detect intact LINEs in genome assemblies, specifically focusing on canFam4 in German Shepherds. The L1EM method is used to determine reads mapping to each LINE-1 locus. The study aims to advance understanding of LINE-1 biology and its implications in disease.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Adaptive Designs with Multiple Objectives Carl-Fredrik Caffe Burman Early Biometrics & Statistical Innovation, Data Science & Artificial Intelligence, R&D, AstraZeneca, Gothenburg, Sweden PSI 1-day meeting on Adaptive Designs and their Application October 13, 2022
Adaptive Designs Adaptive Designs with Multiple Objectives Multiple Objectives 2
0 Preliminaries
Keep it simple! What s important in this trial / project? Does this methodology make sense? Meaningful to medics, patients, payers? Optimal : Real improvements or gains? Measuring value Bayesian & Frequentist Prescriptive or descriptive? 4
Everything is (multi-variate) Normal due to the Central Limit Theorem Transformations sometimes needed Some exceptions to be wary of - Small sample sizes - Discrete data with few events - Outliers Examples MVN - Placebo-adjusted efficacy for two different doses - Interim and Final analysis - All-comers (AC) and Biomarker positive (BM+) subpopulation 5
Some notation Testing null hypothesis ?: ? 0 ? ?2 Efficacy estimate: Variance of estimate: 1 ? = Information ?2 ??= ? ? ? = ? = 11 ? Z score is N(0,1): One-sided p-value: ?1,?2 MVN Two endpoints: Interim & Final analysis: ?1,?2 MVN ? = 2.5% Type 1 Error should be 6
1 Adaptive Designs & Group Sequential Designs
What is an Adaptive Design? Can change design features mid-way. Pre-specify weights for stages Independent under null ?(1) ?(2) ?(1) ?(1) ?(2) ?(2) Inverse normal Weights ?1+ ?2= 1 ?combined ? 8
Any adaptation is OK mathematically but we need to think about interpretability 9
Early winning in Adaptive Designs Group-Sequential Designs (GSDs) is a kind of Adaptive Design (without AD weighting) K planned analysis time points May claim statistical significant efficacy (and typically stop trial) after any of these analyses But have to adjust critical values, so that total Type 1 Error is ? = 2.5%. Same approach possible for more general Adaptive Designs 10
Group Sequential Design (GSD) Independent under null ?1 ?2 1 1 ?1= ?2= ?tot= ?1+ ?2 2 2 ?1 ?2 ?(2)= ?2 ?2 ?1= ?1 ?1 ?1 ?tot, ?2= ?2 ?tot ? = ?1?1+ ?2?2 ?1= ?2= ?1 ?1+ ?(2)?1 11 ? ?1 ? ?tot 1 ?1 ?1 1 ?1,?2 ??? ,
Critical values ?1>?1 ?2> ?2 Claim statistical significance if ?1>?1 at interim analysis OR ?2> ?2 at final analysis Equivalent: ?1<?1 at interim analysis OR ?2< ?2 at final analysis Type 1 Error = ??{?1> ?1} or {?2> ?2} Pre-specify ?1 or, equivalently, ?1= 1(1 ?1) Find ?2 so that ??{?1> ?1} or {?2> ?2} = ? = 2.5% using MVN How aggressively we choose ?1 is quite important. 12
Power (yellow) and Prob(Early win), as functions of interim alpha
Comparing different (group-sequential) designs Fixed GSD GSD Old standard Alternative Interim time NA 24.0 months 24.0 m Prob(WIN early) 0% 25.3% 39.1% Final time 36.0 m 36.2 m 36.7 m Power (total) 90.0% 90.0% 90.0% E[NPV] 90 95.8 98.7 Assume NPV increases by 2 units per month. (100 at 36m, 124 at 24m) NPV=100 at 36.0 m (Index value 100 at fixed design read-out) NPV=124 at 24.0 m (Assume 20% higher if gaining 1 year) NPV=99.6 at 36.2 m (Delay 0.2m = 6days to get same overall power) NPV=98.6 at 36.7 m (Delay 0.7m = 21 days.) Fixed: GSD Old: E[NPV] = 25.3% * 124 + (90%-25.3%) * 99.6 = 95.8 GSD Alternative E[NPV] = 39.1% * 124 + (90%-39.1%) * 98.6 = 98.7 E[NPV] = 90% * 100 = 90
Win early in Adaptive Design ?1= ?(1) ?2= ?(1)?1+?(2)?2 1 ?1 ?1 1 0 0 ?1,?2 ??? , under null hypothesis, just as for GSD but weights may not reflect information in the AD case Claim statistical significance if ?1>?1 at interim analysis OR ?2> ?2 at final analysis 15
Alpha spending Cannot predict exact information at interim and final analyses Can always adjust ?FA at final analysis to control T1E A handy solution is to pre-specify an alpha spending function ? ? , where ? ? is increasing, non-negative and ? = 2.5%. t is usually information (fraction) for a GSD (but could in principle be e.g. calendar time, number of interims made, etc.) t is accumulated weight in an AD Algorithm At 1stanalysis at time ?1, test at nominal level ?1= ?(?1) At k:th analysis at time ??, choose nominal level ?? so that ?? ?=1 ??< ?? = ?(??) ? 16
2 Multiple Objectives
Multiple objectives (For now: Consider fixed, not adaptive design) Multiple endpoints, e.g. CV outcome trial: MACE, CVD, QoL Oncology: PFS, OS Heart Failure: Time-to-first, Number of HF hospitalisations Multiple time points Alzheimer: 26 and 78 weeks Multiple doses PEGASUS trial: ticagrelor 60 mg and 90 mg vs placebo Multiple (sub)populations Respiratory: biomarker positive (eosinophil high) and all-comer populations 18
Family-Wise Error Rate (FWER): Prob of rejecting any true null hypothesis 19
Bonferroni-based Recycling Two operations - Splitting alpha between hypotheses (Bonferroni) - Reuse alpha when winning (cf. Hierarchical procedure) Does not utilise correlation structure 20
Splitting (weighted Bonferroni) ?2 ?1 ?3 ?1 ?2 ?3 21
Recycling: Hierarchical procedure ?1 ?2 22
Can combine Splitting & Recycling v1 v2 v3 ?1 ?1 ?2 ?3 ?4 ?5 ?2 ?3 ?4 ?6 and combined recursively in any number of steps 23
Improving Bonferroni by utilising correlations ?2 ?1 ?3 ?1+ ?2+ ?3= 1 ?1 ?2 ?3 Conservative case: Not using correlations ?? ?1< ?1? ?2< ?2? ?3< ?3? ? Utilising known correlation structure 1 ?12 1 ?13 ?23 1 0 0 0 ?1,?2,?3 MVN , under global null May choose ? ? so that ?? ?1< ?1? ?2< ?2? ?3< ?3? This gives higher power. Example: Dunnett when 2 doses vs. common control = ? 24
Are correlations known? Cf. GSD, where correlations between analysis times will be known at final analysis Multiple arms (e.g. doses) vs same Control: Known correlation (approximately). Dunnett All-comers and biomarker subpopulation: Known correlation (approximately). Spiessens-Debois Multiple endpoints (or multiple times for same endpoint): Unknown correlation 25
Can we estimate correlation between endpoints? Earlier trials Current trial Correlation for variables Bootstrap for test statistics Randomisation test 26
3 Adaptive Designs with Multiple Objectives
Primary & Secondary endpoint in Adaptive Design ??1<?1 ??2< ?2 ??1<? ??2<? When Primary endpoint (P) is stat sign, stop trial and test also Secondary (S) May recycle full ? from P to S But may NOT test at full ? whenever P wins 28
Why could we get Type 1 Error inflation? ??1<?1 ??2< ?2 ??1< ? ??2< ? The problem arises when there is an effect on Primary, but not on Secondary Winning on Primary at interim could give information that Secondary endpoint estimate is random high. Thus, prob to falsely reject Secondary may be inflated. 29
We may test at same level as Primary ??1<?1 ??2< ?2 ??1< ?1 ??2< ?2 How about power for Secondary? Note that signal-to-noise is worse at interim, better at final. If likely to win at interim on Primary, may want to spend more alpha for Secondary at interim 30
What we can do Primary: any alpha spending If and only if winning on Primary recycle full ? to Secondary endpoint Use any alpha spending for Secondary 31
How to think (1) 1. Pre-define a Bonferroni-based recycling multiple testing procedure for all hypotheses in the confirmatory family. 2. Also pre-define, for every hypothesis ??, an alpha spending function, ??(?, ??), where ?? is the test mass allocated to ?? from the recycling procedure. The alpha spending function will be increasing in its argument and fulfil ??(?, ??) ?? for all t. When analysing the trial: 1. For each hypothesis and interim/final analysis time point, test based on the alpha spending function for that hypothesis. 2. Whenever a null hypothesis is rejected, recycle all alpha associated with this hypothesis to other hypotheses based on the pre-specified recycling scheme. 32
Example 1. ? 2 = 1.25% is allocated to MACE for each dose 0.5 0.5 2. This is spent using O Brien-Fleming. Low dose MACE High dose MACE 3. When MACE is sign for one dose, this dose is stopped. 4. If MACE is sign for one dose, QoL for the same dose. Pocock alpha spending is used for QoL. Can only be tested at same time as MACE was sign. ? 2 = 1.25% is recycled to Low dose QoL High dose QoL 5. If QoL is sign for one dose, MACE for the other dose can now be tested at full ?. All of the extra alpha is spent at the final analysis.
How to think (2) 1. Pre-define a Bonferroni-based recycling multiple testing procedure for all hypotheses in the confirmatory family. 2. Also pre-define, for every hypothesis ??, an alpha spending function, ??(?, ??), where ?? is the test mass allocated to ?? from the recycling procedure. The alpha spending function will be increasing in its argument and fulfil ??(?, ??) ?? for all t. 3. Add correlations to improve on the above. May always use closed testing to check that the total procedure controls FWER strongly. 34
Discussion topics (Use a societal perspective!) Should we continue trials after winning? Should we test null hypotheses that are obviously wrong? Should we (regulatory agencies) request strong FWER control? Weak control? 35
Confidentiality Notice This file is private and may contain confidential and proprietary information. If you have received this file in error, please notify us and remove it from your system and note that you must not copy, distribute or take any action in reliance on it. Any unauthorized use or disclosure of the contents of this file is not permitted and may be unlawful. AstraZeneca PLC, 1 Francis Crick Avenue, Cambridge Biomedical Campus, Cambridge, CB2 0AA, UK, T: +44(0)203 749 5000, www.astrazeneca.com 36