Lipid Reactions Overview: Dehydration, Hydrolysis, Hydrogenation, Dehydrogenation
Explore important lipid reactions such as dehydration, hydrolysis, hydrogenation, and dehydrogenation. Understand how molecules are formed and broken apart, and learn to complete reactions given the products. Enhance your skills in lipid chemistry with a focus on key reactions.
Uploaded on | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
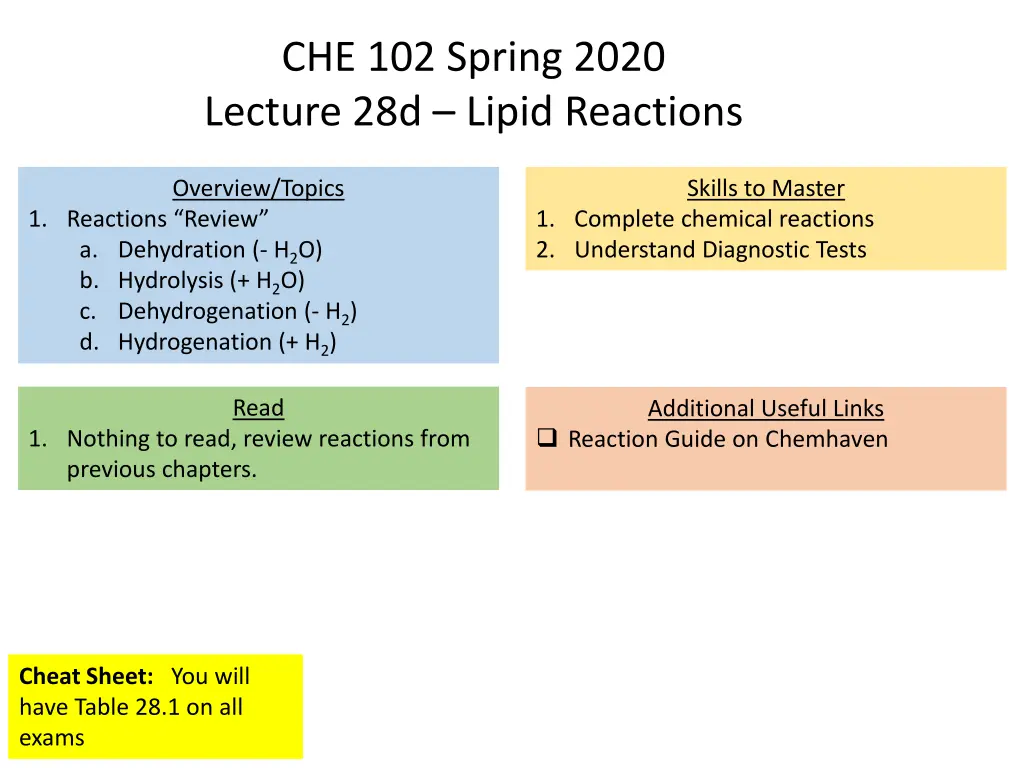
CHE 102 Spring 2020 Lecture 28d Lipid Reactions Overview/Topics Skills to Master 1. Reactions Review a. Dehydration (- H2O) b. Hydrolysis (+ H2O) c. Dehydrogenation (- H2) d. Hydrogenation (+ H2) 1. Complete chemical reactions 2. Understand Diagnostic Tests Read Additional Useful Links Reaction Guide on Chemhaven 1. Nothing to read, review reactions from previous chapters. Cheat Sheet: You will have Table 28.1 on all exams
Dehydration Dehydration reactions are how most molecules in this chapter are formed Glycerol + 3 FA Fats and Oils CA + Alcohol Waxes Glycerol + 2 FA + Phosphate + Amino-alcohol Phospholipid Etc Can you Complete the reaction given the products?
Hydrolysis 1. Hydrolysis reactions are how most molecules in this chapter are broken apart 2. Opposite of Dehydration Can you Complete the reaction given the products?
Hydrogenation 1. Addition reaction, add H2 across a C=C double bond 2. Unsaturated FA Saturated FA +H2 OH OH H H3 C O H3 C O H 1. Review Ch. 20 and Lab - Hydrocarbons 2. Diagnostic Test Can determine the relative number of C=C in a molecule 3. Color change is proportional to number of C=C bonds 4. Modification in lab we use Sudan III Br + Br2 (Fast) Br Orange Clear Can you Complete the reaction given the products? Diagnostic Test + Br2 Slow (Requires UV light) Orange NR
Dehydrogenation 1. Elimination Reaction, remove H2 to create C=C double bond 2. Saturated FA Unsaturated FA 3. Will see in again in Ch. 34/35 OH -H2 OH H H3 C O H3 C O H Can you Complete the reaction given the products?