Long-term Analysis of B/F/TAF Treatment in HIV-Positive Adults
This study provides a comprehensive analysis of the long-term effectiveness and safety of B/F/TAF treatment in treatment-naive adults living with HIV over a four-year follow-up period. The research discusses participant disposition, baseline characteristics, study designs, and key inclusion criteria in detail. Findings shed light on the efficacy of B/F/TAF in maintaining viral suppression and managing the disease in this patient population through various endpoints.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Long-term Analysis of B/F/TAF in Treatment-Na ve Adults Living With HIV Through Four Years of Follow-up Jose Arribas,1Chloe Orkin,2Franco Maggiolo,3Andrea Antinori,4Adriano Lazzarin,5 Yazdan Yasdanpanah,6Hans-J rgen Stellbrink,7Anton Pozniak,8Edwin DeJesus,9 Hailin Huang,10Rima Acosta,10Diana Brainard,10Jason Hindman,10Hal Martin,10 Paul Sax11 on behalf of the Study 1489 and 1490 Investigators 1Hospital Universitario La Paz, Madrid, Spain; 2Ambrose King Centre, Royal London Hospital, London, UK; 3ASST Papa Giovanni XXIII, Bergamo, Italy; 4Istituto Nazionale Malattie Infettive, Lazzaro Spallanzani, Rome, Italy; 5Ospedale San Raffaele, Milano, Italy; 6H pital Bichat Claude-Bernard, Paris, France; 7ICH Study Center, Hamburg, Germany; 8Chelsea and Westminster Hospital, London; 9Orlando Immunology Center, Orlando, Florida, USA; 10Gilead Sciences, Inc., Foster City, California, USA; 11Brigham and Women s Hospital, Boston, Massachusetts, USA IAS 2021, 18 21 July: PEB151, 2137
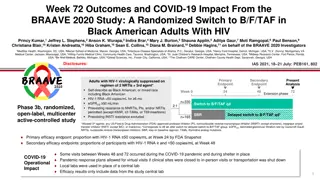
Study Designs: Randomized, Double Blind, Active Controlled 2 Endpoint 1 Endpoint 2 Endpoint 2 Endpoint Week 0 48 96 144 192 240 Treatment-Na ve Adults B/F/TAF qd n=314 Study 1489 HLA B*5701 negative Negative for chronic HBV eGFRCG 50 mL/min DTG/ABC/3TC placebo qd 1:1 Open-label B/F/TAF DTG/ABC/3TC qd n=315 B/F/TAF placebo qd Key inclusion criteria for both: No known resistance to FTC, TAF, ABC, or 3TC HIV-1 RNA 500 copies/mL B/F/TAF qd n=320 DTG + F/TAF placebo qd Study 1490 Chronic HBV or HCV infection allowed eGFRCG 30 mL/min 1:1 Open-label B/F/TAF DTG + F/TAF qd n=325 B/F/TAFplacebo qd 3TC, lamivudine; ABC, abacavir; eGFRCG, estimated glomerular filtration rate by Cockcroft-Gault equation; HBV, hepatitis B virus; HCV, hepatitis C virus; HLA, human leukocyte antigen. 3
Participant Disposition Through Week 192 and Baseline Characteristics Participant Disposition Through Week 192 Baseline Characteristics Pooled B/F/TAF n=634 Randomized: N=1288 Randomized Phase Median age, y (range) 32 (18 71) DTG + F/TAF: n=330 B/F/TAF: n=643 DTG/ABC/3TC: n=315 Female at birth, n (%) 69 (11) Randomized, not treated: n=9 Randomized, not treated: n=5 Race/ethnicity, n (%) B/F/TAF: n=634 DTG/ABC/3TC: n=315 DTG + F/TAF: n=325 Black or African descent 211 (33) Premature D/C: n=115 (18%)* Hispanic/Latinx ethnicity 155 (24) Did not enter OLE: n=13 (2%) Median body weight, kg (Q1, Q3) 77 (68, 88) Median body mass index, kg/m2 (Q1, Q3) 25.1 (22.3, 28.6) Open Label B/F/TAF: n=506 Median HIV-1 RNA, log10copies/mL (Q1, Q3) 4.4 (4.0, 4.9) Reason for D/C, n* Participant decision Lost to follow-up AE Death Protocol violation Noncompliance 25 (5%) 12 9 1 1 1 1 HIV-1 RNA >100,000 copies/mL, n (%) 119 (19) Median CD4 cells/ L (Q1, Q3) 442 (293, 590) OLE CD4 count <200 cells/ L, n (%) 80 (13) Asymptomatic HIV infection, n (%) 572 (90) Median eGFRCG, mL/min (Q1, Q3) 122 (104, 143) On B/F/TAF to Data Cut Date: n=481 *In the randomized phase n=115 participants prematurely discontinued (D/C) study drug: 6 (1%) due to adverse event (AE), 0 due to lack of efficacy, and 109 (17%) due to other reasons; of the 506 who entered the OLE, 25 prematurely D/C study drug: 1 (<1%) due to AE, 0 due to lack of efficacy, and 24 (5%) due to other reasons. CD4, cluster of differentiation-4; Q, quartile. 4
Virologic Outcomes and Resistance HIV-1 RNA <50 Copies/mL CD4 Change From Baseline 100 99 99 99 99 99 99 98 Median Change From Baseline, 100 500 94 92 Cells/ L (Q1, Q3) 80 400 88 87 Participants, % 85 83 79 75 60 300 +289 40 200 20 100 Missing=excluded Missing=failure 0 0 0 48 96 144 192 0 24 48 72 96 120 144 168 192 Week Week M=E: n=* 634 607 589 564 557 543 531 505 480 n= 634 584 546 517 475 99% of B/F/TAF participants maintained HIV-1 RNA <50 copies/mL (M=E at Week 192) At Week 192, 476 participants had HIV-1 RNA <50 copies/mL and 4 participants had HIV-1 RNA >50 copies/mL All B/F/TAF Week 96 7 0 0 Participants, n Met criteria for resistance testing NRTI resistance detected INSTI resistance detected Week 48 8 0 0 Week 144 8 0 0 Week 192 8 0 0 No participant failed with resistance to any component of B/F/TAF *Participants with nonmissing HIV-1 RNA value; While receiving B/F/TAF (observed data); Resistance testing performed for participants with confirmed HIV-1 RNA 50 copies/mL with the confirmation visit having 200 copies/mL or 200 copies/mL at last visit, without resuppression of HIV-1 RNA to <50 copies/mL while on study drug. INSTI, integrase strand transfer inhibitor; M=E, missing=excluded; M=F, missing=failure; NRTI, nucleoside reverse-transcriptase inhibitor. 5
Treatment-Emergent AEs and AEs Leading to Discontinuation Through Week 192* B/F/TAF Overall: n=634 (Baseline Week 192) 94 B/F/TAF OLE: n=506 (Week 144 192) 70 B/F/TAF: n=634 Participants, % Any AE AEs 10% overall Diarrhea Headache Nasopharyngitis URTI Syphilis Arthralgia Back pain Nausea Cough Fatigue Insomnia Influenza Any study drug-related AE Study drug-related AEs 2% overall Headache Diarrhea Nausea Fatigue Dizziness Insomnia Cardiac arrest (Week 4; Day 28) AEs leading to D/C Not related n=3 (<1%) Paranoia (Week 42; Day 299) 21 18 18 16 15 13 13 13 13 11 10 10 28 4 4 6 5 5 3 4 3 5 2 2 3 2 Intervertebral discitis (Week 195; Day 1366) Chest pain (Week 0; Day 1) Abdominal distension (Week 0; Day 1) AEs leading to D/C Study drug related n=4 (1%) Sleep disorder, dyspepsia, and tension headache (Week 2; Day 15); depressed mood and insomnia (Week 9; Day 63) Depression (Week 48; Day 337) Cardiac arrest (Week 4; Day 28) Poorly differentiated gastric adenocarcinoma (Week 53; Day 376) Hypertensive heart disease (Week 58; Day 412) 5 5 4 3 2 2 <1 <1 <1 <1 <1 0 Deaths n=6 (1%) Self-inflicted wrist wound (Week 93; Day 656) Combined toxicity of chloroethane and methamphetamine (Week 110; Day 771) Sudden cardiac arrest (Week 151; Day 1060) *Red shading indicates AEs occurring during OLE. URTI, upper respiratory tract infection. Few AEs leading to B/F/TAF D/C were observed through 4 y of follow-up Only a small proportion of AEs leading to D/C occurred after Year 1 6
eGFRCG and Weight Changes eGFRCG Weight 5 8 Median Change From Baseline, +0.7 4 Median Change, kg/y Week 192 Week 144 Week 96 Week 48 +0.7 0 mL/min (Q1, Q3) +0.5 0.3 3 -8 -5.8 0.9 0.4 -7.5 -8.0 2 -8.8 +3.0 -16 1 -24 3 0 0 48 96 144 192 Week No reported cases of proximal renal tubulopathy or D/Cs due to renal AEs were observed on B/F/TAF Initial decline followed by stable eGFRCG are consistent with inhibition of tubular creatinine secretion via organic cation transporter-2 by BIC Most of the weight change took place in the first year, followed by annual changes of +0.5 0.7 kg/y 7
Conclusions In treatment-na ve people living with HIV, through 4 y of follow-up among those originally randomized to B/F/TAF, we observed: High rates of virologic suppression with no treatment-emergent resistance Few AEs leading to D/C, no renal related D/Cs overall, and AEs were rare between Weeks 144 192 9/634 participants (1%) experienced a treatment-emergent drug-related AE during the OLE Small changes in lipid fractions with minor change from baseline in TC:HDL ratio at Week 192 Weight gain of ~3 kg in the first year, followed by annual changes of +0.5 0.7 kg/y, consistent with data from previous studies in treatment-na ve populations, and similar to findings in the general population of increases in body mass index and weight of nearly 1 kg/y1-9 These results confirm the long-term safety and efficacy of B/F/TAF 1. Hill JO, et al. Science 2003;299:853-5; 2. Lakey W, et al. AIDS Res Hum Retroviruses 2013;29:435-40; 3. Sax PE, et al. Clin Infect Dis 2020;71:1379-89; 4. Sharma A, et al. PLoS One 2015;10:e0143740; 5. Taramasso L, et al. Open Forum Infect Dis 2017;4:ofx239; 6. Tate T, et al. Antivir Ther 2012;17:1281-9; 7. WHO 2016. Centers for Disease Control and Prevention. Obesity and overweight. 2016; 8. Workowski K, et al. CROI 2021, science spotlight 2268; 9. Yuh B, et al. Clin Infect Dis 2015;60:1852-9. 8