LPV/r Monotherapy Study: MOST Trial Outcomes
Explore the outcomes of the MOST study regarding the switch to LPV/r monotherapy in HIV patients, focusing on virologic failure, clinical symptoms, CD4 cell counts, and CSF analysis after a median follow-up of 48 weeks. The study aimed to assess the non-inferiority of LPV/r monotherapy compared to continued ART therapy in maintaining HIV-1 RNA levels below 50 c/mL. Results showed virologic failure in 6 out of 29 patients in the monotherapy group, with clinical symptoms at failure and low nadir CD4 cell counts, but no marked elevation of HIV-1 RNA in genital secretions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
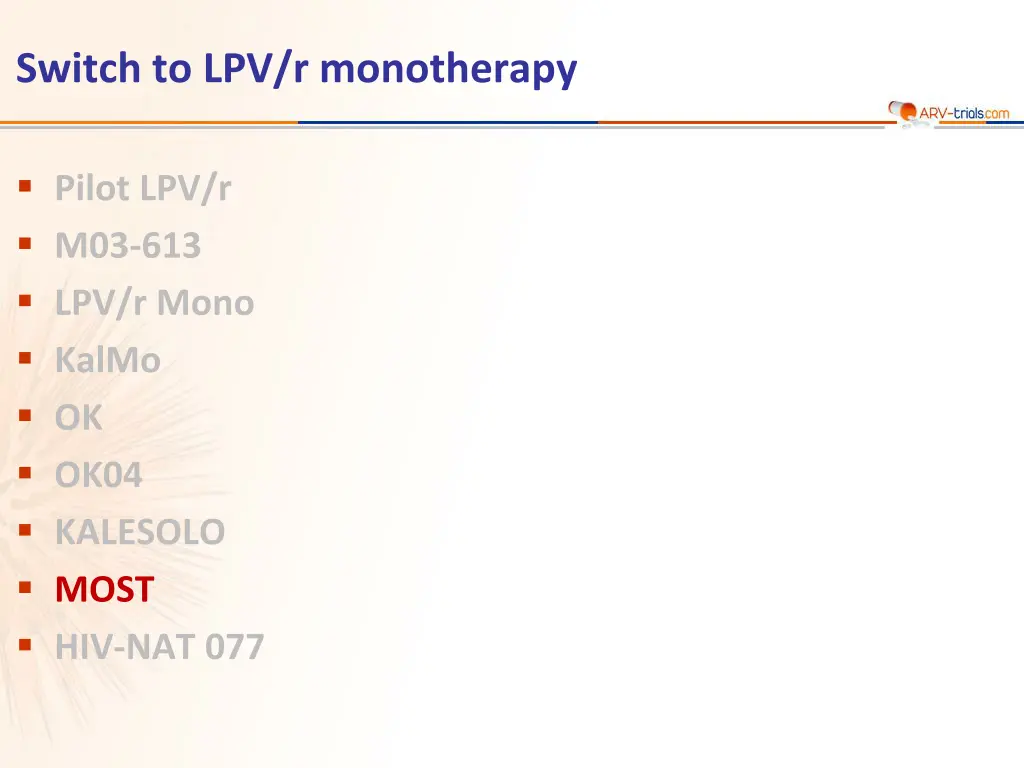
Switch to LPV/r monotherapy Pilot LPV/r M03-613 LPV/r Mono KalMo OK OK04 KALESOLO MOST HIV-NAT 077
MOST Study: Switch to LPV/r monotherapy Design Randomisation 1 : 1 Open-label W96 Continuation current antiretroviral therapy (cART)* N = 31 Target of 100 HIV+ On cART > 6 months HIV-1 RNA < 50 c/mL > 3 months Baseline determination of HIV-1 RNA in CSF and genital secretions N = 29 LPV/r 400/100 mg bid * Possibility offered to switch to LPV/r monotherapy at W48 delayed switch) Objective Non inferiority of the monotherapy group in the proportion of patients with HIV-1 RNA < 50 c/mL in the plasma and treatment failure in the CNS or the genital compartment without modification of treatment (per-protocol analysis) ; lower limit of CI for the difference = - 12%, 80% power Study was prematurely stopped before full recruitment when 6 patients on monotherapy (none in cART group) demonstrated a virologic failure in blood Gutmann C, AIDS 2010;24:2347-54 MOST
MOST Study: Switch to LPV/r monotherapy Baseline characteristics Continuation of cART N = 31 LPV/r monotherapy N = 29 Mean age, years Female CDC stage C CD4 cell count at baseline, median/mm3 CD4 cell count at nadir, median/mm3 HIV-RNA set point, mean log10c/ml ARV at inclusion 46 23% 23% 465 160 4.7 42 34% 34% 498 160 4.9 PI-based 74% 23% 73% 24% NNRTI-based 3% 3% 3 NRTIs Gutmann C, AIDS 2010;24:2347-54 MOST
MOST Study: Switch to LPV/r monotherapy Outcome Median follow-up: 48 weeks Virologic failure (2 consecutive plasma HIV-1 RNA > 400 c/mL) occurred in 6/29 patients in the LPV/r monotherapy group, after a median of 12 weeks, vs 0/31 in the continued antiretroviral therapy group In these 6 failures, the median duration of HIV-1 RNA < 50 c/mL was 50 months ; 5/6 patients had clinical symptoms at the time of failure, all symptoms resolving after treatment switch ; all 6 patients had a nadir CD4 cell count < 200/mm3 CSF was examined in 45 patients at study termination (25 on LPV/r monotherapy and plasma HIV-1 RNA < 400 c/mL, 5 failing monotherapy and 15 continuing prior ARV therapy with plasma HIV-1 RNA < 50 c/mL) CSF HIV-1 RNA was > 40 c/mL 8/25 patients on monotherapy none of the 15 patients still on continued treatment (p = 0.01) No marked elevation of HIV-1 RNA in the genital secretions Gutmann C, AIDS 2010;24:2347-54 MOST
MOST Study: Switch to LPV/r monotherapy Patients with treatment failure in blood or detection of elevated HIV-1 RNA in CSF Week on study/on monotherapy HIV-1 RNA plasma, log10c/ml 4.3 4.2 4.1 HIV-1 RNA CSF, log10c/mL 5.1 3.1 5.0 CD4 nadir /mm3 Treatment arm Sex Pre-Treatment 1 2 3 Male Female Female TDF/FTC/ATV/r ZDV/3TC/LPV/r ABC/3TC/LPV/r 57 5 149 LPV/r mono Delayed Switch LPV/r mono 12 Blood failure (HIV-1 RNA > 400 c/mL) 60/12 12 4 5 6 Male Male Female ZDV/3TC/EFV TDF/3TC/LPV/r TDF/3TC/EFV 7 54 160 LPV/r mono LPV/r mono LPV/r mono 24 6 24 3.0 5.0 3.0 4.1 ND 3.7 1 2 3 4 Male Male Female Male TDF/FTC/LPV/r TDF/3TC/ATV/r ABC/3TC/LPV/r TDF/3TC/ZDV/EFV 211 370 100 130 Delayed Switch Delayed Switch LPV/r mono Delayed Switch 96/48 66/18 63 68/20 < 1.6 2.2 2.3 2.1 2.9 3.4 4.3 3.4 HIV-RNA detectable in CSF Monotherapy arm 5 6 7 8 9 10 11 12 Male Male Female Female Male Male Male Male ZDV/3TC/LPV/r TDF/FTC/LPV/r ABC/3TC/ZDV/LPV/r ZDV/3TC/LPV/r TDF/FTC/LPV/r TDF/FTC/ATV/r TDF/3TC/EFV TDF/3TC/ATV/r 120 20 220 17 20 126 185 370 Delayed Switch LPV/r mono LPV/r mono LPV/r mono cART at baseline cART at baseline cART at baseline Delayed Switch 72/24 37 48 44 0 0 0 48/0 < 1.6 < 1.6 1.9 < 1.6 < 1.6 < 1.6 < 1.6 < 1.6 2.1 2.4 2.5 1.9 1.6 1.7 1.9 1.6 HIV-RNA detectable in CSF Continuation therapy arm Gutmann C, AIDS 2010;24:2347-54 MOST
MOST Study: Switch to LPV/r monotherapy Conclusions Maintenance of HIV treatment with LPV/r monotherapy should not be recommended as a standard strategy ; this is particularly evident in patients with a CD4 cell count < 200/mm3at nadir The proportion of patients with detectable HIV-1 RNA in CSF was not only significantly higher on LPV/r monotherapy than on continued combination therapy (32% vs 0% ; p = 0.01), but the difference appears biologically (CSF inflammation) and clinically (acute symptoms) relevant Gutmann C, AIDS 2010;24:2347-54 MOST




![[PDF] DOWNLOAD READ Diagnosis Solving the Most Baffling Medical Mysterie](/thumb/9855/pdf-download-read-diagnosis-solving-the-most-baffling-medical-mysterie.jpg)