Luminescence and Photosensitization in Chemistry
Learn about the fascinating phenomena of luminescence, including chemiluminescence, fluorescence, and phosphorescence. Discover how certain substances emit light through chemical reactions or exposure to radiation. Dive into the excitation of electrons in fluorescence and phosphorescence, and explore photosensitization reactions where molecules transfer energy to induce photochemical processes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
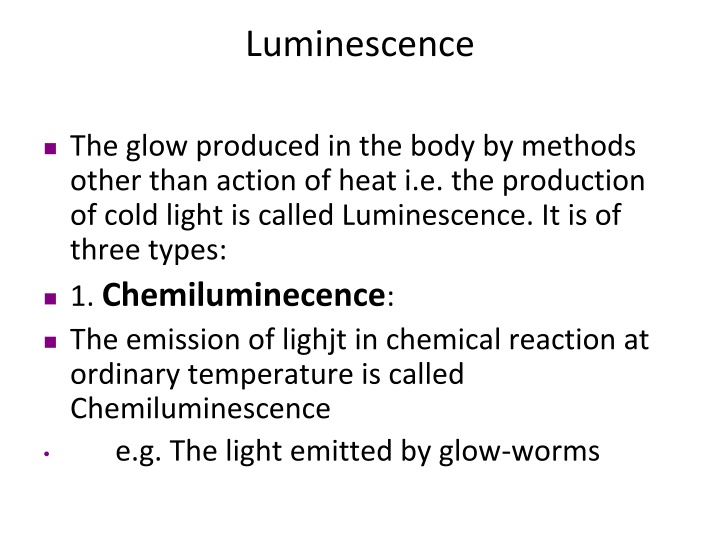
Luminescence The glow produced in the body by methods other than action of heat i.e. the production of cold light is called Luminescence. It is of three types: 1. Chemiluminecence: The emission of lighjt in chemical reaction at ordinary temperature is called Chemiluminescence e.g. The light emitted by glow-worms
Fluorescence: Certain substances when exposed to light or certain other radiations absorb the energy and then immediately start re- emitting the energy. Such substances are called fluorescent substances and the phenomenon is called fluorescence . e.g Organic dyes such as eosin,fluorescein etc. vapour of sodium,mercury,iodine etc. Phosphorescence: There are certain substances which continue to glow for some time even after the external light is cut off. Thus, phosphorescence is a slow fluorescence.
Fluorescence and phosphorescence in terms of excitation of electrons: Singlet excited state S1 (pair of electorns with Opposite spins but each in different orbital) Triplet excited state T1 (pair of electrons with parallel spins in different Orbitals) Singlet ground state So The exicted species can return to the ground state by losing all of its excess energy by any one of the paths shown in jablonski diagram.
Photosensitisation Photosensitized reactions: An electronically excited molecule can transfer its energy to a second species which then undergoes a photochemical process even though it was not itself directly excited. Mercury acting as a photosensitizer: Hg+hv Hg* Hg*+H2 Hg+2H Chlorophyll acting as a photosensitizer CO2+H2O+hv 1/6(C6H12O6)+O2 chlorophyll