Lung Cancer Treatment Trials and Case Discussion
Explore clinical trials comparing different treatments for advanced non-small cell lung cancer (NSCLC) with ALK or EGFR mutations. Learn about the efficacy and adverse events associated with alectinib, crizotinib, afatinib, and gefitinib. Delve into a case discussion involving a 68-year-old man with NSCLC to determine the optimal treatment approach. Additionally, discover ongoing long-term follow-up trials for patients with specific genetic mutations in NSCLC.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
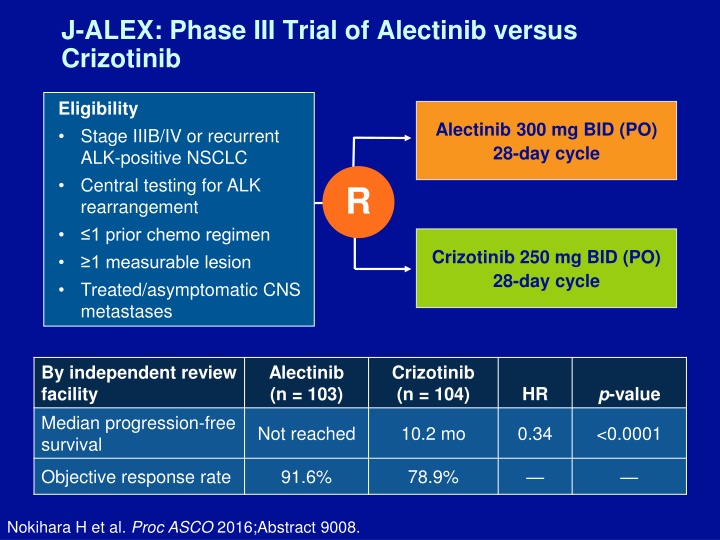
J-ALEX: Phase III Trial of Alectinib versus Crizotinib Eligibility Stage IIIB/IV or recurrent ALK-positive NSCLC Central testing for ALK rearrangement 1 prior chemo regimen 1 measurable lesion Treated/asymptomatic CNS metastases Alectinib 300 mg BID (PO) 28-day cycle R Crizotinib 250 mg BID (PO) 28-day cycle By independent review facility Median progression-free survival Alectinib (n = 103) Crizotinib (n = 104) p-value HR Not reached 10.2 mo 0.34 <0.0001 Objective response rate 91.6% 78.9% Nokihara H et al. Proc ASCO 2016;Abstract 9008.
J-ALEX: Adverse Events Occurring in 20% of Patients All grades Grade 3 or 4 Alectinib (N = 103) 36 (35.0%) Crizotinib (N = 104) 46 (44.2%) Alectinib (N = 103) 1 (1.0%) Crizotinib (N = 104) 1 (1.0%) Constipation Nausea 11 (10.7%) 77 (74.0%) 0 2 (1.9%) Diarrhea 9 (8.7%) 76 (73.1%) 0 2 (1.9%) Vomiting 6 (5.8%) 60 (57.7%) 0 2 (1.9%) Visual disturbance 1 (1.0%) 57 (54.8%) 0 0 Nokihara H et al. Proc ASCO 2016;Abstract 9008.
LUX-Lung 7: Phase IIb Trial of First-Line Afatinib versus Gefitinib in EGFR Mutation-Positive Advanced NSCLC HR (p-value) Afatinib Gefitinib Median PFS 11.0 mo 10.9 mo 0.73 (0.017) Time to treatment failure 13.7 mo 11.5 mo Not reported Objective response rate 70% 56% Not reported Median duration of response 10.1 mo 8.4 mo Not reported Afatinib treatment led to improved outcomes regardless of the mutation subtype, with benefits observed in participants harboring the exon 19 deletion and in those with the L858R EGFR mutation. Park K et al. Proc ESMO 2015;Abstract LBA2_PR; Hirsh V et al. Proc ASCO 2016;Abstract 9046.
Case Discussion A 68-year-old man and former smoker with Stage IIIA lung cancer LUL mass with mediastinal lymph node involvement Biopsy-proven pan-wild-type NSCLC Should you give neoadjuvant treatment first, or should you just go right into concurrent chemo/RT?
ALCHEMIST EGFR Erlotinib 150 mg PO BID x 2 y Long-term follow-up Treatment Trial A081105 Patients with tumors with an EGFR mutation R Long-term follow-up Placebo PO BID x 2 y ALK Resected NSCLC tissue tested on ALCHEMIST Screening Trial Crizotinib 250 mg PO BID x 2 y Long-term follow-up Treatment Trial EA4512 Patients with tumors with an ALK rearrangement R Long-term follow-up Placebo PO BID x 2 y Nivolumab Treatment Trial EA5142 Long-term follow-up Patients with tumors with wild-type EGFR and ALK Nivolumab x 1 y R Long-term follow-up Observation
Case Discussion A 68-year-old man and former smoker with Stage IIIA lung cancer LUL mass with mediastinal lymph node involvement Biopsy-proven pan-wild-type NSCLC Should neoadjuvant treatment be administered first, or should you start immediately with chemoradiation therapy? Received neoadjuvant chemotherapy with pemetrexed/carboplatin chemoradiation therapy
Phase III PROCLAIM Trial Design Recovery period Concurrent phase Consolidation phase Eligibility (n = 598) Pemetrexed, cisplatin, TRT 3 cycles (n = 283) Arm A Pemetrexed 4 cycles Previously untreated Stage IIIA-IIIB nonsquamous NSCLC PS 0/1 R PR/CR/SD per RECIST Etopside, cisplatin, TRT 2 cycles (n = 272) Investigator s choice: Etoposide/cisplatin or Vinorelbine/cisplatin or Paclitaxel/carboplatin 2 cycles Arm B TRT = thoracic radiation therapy Senan S et al. J Clin Oncol 2016;34(9):953-62.
PROCLAIM: Efficacy Pem/cis (n = 301) Eto/cis (n = 297) p-value HR Median overall survival 26.8 mo 25.0 mo 0.98 0.831 Median PFS 11.4 mo 9.8 mo 0.86 0.130 Objective response rate 35.9% 33.0% 0.458 The pemetrexed/cisplatin arm had a significantly lower incidence of any drug-related Grade 3 or 4 adverse events: 64.0% versus 76.8%; p = 0.001 Senan S et al. J Clin Oncol 2016;34(9):953-62.
Case Discussion A 68-year-old man and former smoker with Stage IIIA lung cancer LUL mass with mediastinal lymph node involvement Biopsy-proven pan-wild-type NSCLC Should neoadjuvant treatment be administered first, or should you start immediately with chemoradiation therapy? Received neoadjuvant chemotherapy with pemetrexed/carboplatin chemoradiation therapy
Immune Checkpoint Inhibitors as Second-Line Therapy I won t have a blanket statement about this kind of situation, but I really will look at the individual patient If I get a sense that the patient s unlikely going to have a benefit from further chemotherapy because the pemetrexed/platinum is probably one of the better combinations that we have If they are resistant to that, then putting on another taxane- based chemotherapy is unlikely to benefit the patient as much. So those are the patients with whom I may discuss the use of immunotherapy. Tony SK Mok, MD
Personal Approach to the Selection of Immune Checkpoint Inhibitors I don t believe the biomarker is doing a good job in selection. Right now, the overall response rate is 20%. The patients with high expression may have a slightly higher response rate. And then the patients with negative expression may have a lower response rate, but it doesn t mean that they don t respond. So, in a way, even for the patient with negative expression totally negative the efficacy will be equal to docetaxel but not worse than docetaxel. So what are we selecting? Tony SK Mok, MD
Case Discussion A 56-year-old woman and never smoker presents with a typical RUL mass with multiple positive nodules Biopsy-proven TTF1-positive, EGFR exon 19-positive metastatic adenocarcinoma of the lung Receives first-line tyrosine kinase inhibitor (TKI) therapy
LUX-Lung 7: Phase IIb Trial of First-Line Afatinib versus Gefitinib for EGFR Mutation-Positive Advanced NSCLC HR (p-value) Afatinib Gefitinib Median PFS 11.0 mo 10.9 mo 0.73 (0.017) Time to treatment failure 13.7 mo 11.5 mo Not reported Objective response rate 70% 56% Not reported Median duration of response 10.1 mo 8.4 mo Not reported Overall survival data were not mature at the time of data analysis. Park K et al. Proc ESMO 2015;Abstract LBA2_PR; Hirsh V et al. Proc ASCO 2016;Abstract 9046.
Case Discussion A 56-year-old woman and never smoker presents with a typical RUL mass with multiple positive nodules Biopsy-proven TTF1-positive, EGFR exon 19-positive metastatic adenocarcinoma of the lung Receives first-line TKI therapy with gefitinib Patient experienced a great response to gefitinib for 14 months but then experienced disease progression and a pleural effusion; cytology-proven T790M mutation
TIGER-X: Plasma, Tissue and Urine Tests Identify Unique and Overlapping Subsets of Patients with T790M-Positive NSCLC T790M-Positive Cases Tissue positive: 146/181 (81%) Urine Tissue Plasma positive: 145/181 (80%) 16 5 8 Urine positive: 144/181 (80%) 104 104 (57%) were positive by all 3 18 19 sample types 4 Plasma Plasma sensitivity = 80.9% with tissue as reference Urine sensitivity = 81.1% with tissue as reference Wakelee HA et al. Proc ASCO 2016;Abstract 9001.
Case Discussion A 56-year-old woman and never smoker presents with a typical RUL mass with multiple positive nodules Biopsy-proven TTF1-positive, EGFR exon 19-positive metastatic adenocarcinoma of the lung Receives first-line TKI therapy with gefitinib, with great response for 14 months Experiences disease progression and a pleural effusion and has a cytology-proven T790M mutation Received chemotherapy maintenance pemetrexed because osimertinib was not yet approved at the time
Case Discussion A 56-year-old woman and never smoker presents with a typical RUL mass with multiple positive nodules Biopsy-proven TTF1-positive, EGFR exon 19-positive metastatic adenocarcinoma of the lung Receives first-line TKI therapy with gefitinib, with great response, living a normal life Experiences disease progression and a pleural effusion and has a cytology-proven T790M mutation Receives chemotherapy maintenance pemetrexed because osimertinib not yet approved Upon second disease progression she received osimertinib
AURA: Phase I Trial of Osimertinib as First-Line Therapy for EGFR Mutation-Positive Advanced NSCLC Osimertinib 80 mg (n = 30) Osimertinib 160 mg (n = 30) Outcome Median PFS Not reached 19.3 mo Overall response rate 87% 67% Disease control rate 97% Median duration of response Not reached Osimertinib was well tolerated with few adverse events, particularly at the approved 80-mg dose, with which only 10% of patients required dose reduction to manage toxicities. Ramalingam S et al. Proc ESMO 2016;Abstract LBA1_PR.
Phase III IMPRESS Trial NCT01544179 Gefitinib + cisplatin + pemetrexed (n = 133) Eligibility (n = 265) Locally advanced or metastatic NSCLC EGFR mutation-positive Acquired resistance to first-line gefitinib R Placebo + cisplatin + pemetrexed (n = 132) Gefitinib (n = 133) 5.4 mo Placebo (n = 132) 5.4 mo p-value 0.273 HR 0.86 Median PFS Soria JC et al. Lancet Oncol 2015;16(8):990-8; Mok TSK et al. Proc ESMO 2014;Abstract LBA2_PR.
Case Discussion A 56-year-old woman and never smoker presents with a typical RUL mass with multiple positive nodules Biopsy-proven TTF1-positive, EGFR exon 19-positive metastatic adenocarcinoma of the lung Receives first-line TKI therapy with gefitinib, with great response, living a normal life Experiences disease progression and a pleural effusion and has a cytology-proven T790M mutation Receives chemotherapy maintenance pemetrexed because osimertinib not yet approved Upon disease progression she received osimertinib
Frequency of MET Exon 14 Splice Site Mutation in NSCLC Subgroups MET Exon 14 (n = 687) Histology Adenocarcinoma 2.6% Sarcomatoid carcinoma 31.8% Squamous, large cell or lymphoepithelioma- like carcinoma 0 Adenosquamous 4.8% Tong JH et al. Clin Cancer Res 2016;15(12):3048-56.
Treatment Options After Disease Progression on Osimertinib Recently developed mutant-selective irreversible inhibitors are highly active against the T790M mutation, but their efficacy can be compromised by acquired mutation of C797 EAI045, a fourth-generation EGFR inhibitor, is an allosteric inhibitor that targets selected drug-resistant EGFR mutants but spares the wild-type receptor This compound inhibits L858R/T790M-mutant EGFR with low nanomolar potency Jia Y et al. Nature 2016;534(7605):129-32.
Case Discussion A 55-year-old man with a diagnosis of epithelioid mesothelioma who underwent right decortication followed by talc pleurodesis in 2011 After his disease recurred he received pemetrexed/carboplatin followed by repeat thoracotomy decortication with more extensive resection of his diaphragm and reconstruction He experienced a response for 3 years
Case Discussion A 55-year-old man with a diagnosis of epithelioid mesothelioma who underwent right decortication followed by talc pleurodesis in 2011 After his disease recurred he received pemetrexed/carboplatin followed by repeat thoracotomy decortication with more extensive resection of his diaphragm and reconstruction He experienced a response for 3 years Upon disease progression he received pemetrexed/cisplatin with or without cediranib on a clinical trial
Phase II/III IFCT-GFPC-0701 (MAPS) Trial Eligibility (n = 448) Biopsy-proven MPM WHO PS 0-2 No cardiovascular comorbidity Chemotherapy na ve No CNS metastases Pemetrexed + cisplatin 6 cycles R Bevacizumab + pemetrexed + cisplatin 6 cycles Maintenance bev Pem/Cis (n = 225) Bev/Pem/Cis (n = 223) p-value Outcome HR Median OS 16.07 mo 18.82 mo 0.76 0.0127 Median PFS 7.48 mo 9.59 mo 0.61 <0.0001 MPM = malignant pleural mesothelioma Zalcman G et al. Proc ASCO 2015;Abstract 7500.
Phase I Trial of Anti-Mesothelin Antibody Anetumab Ravtansine Eligibility (n = 147) Solid tumor Refractory to chemotherapy Anetumab ravtansine MTD = 6.5 mg/kg q3wk Patients with MPM treated at MTD (n = 16): Objective tumor shrinkage, 5/16 (31%) Stable disease, 7/16 (44%) Those who received the agent as second-line therapy (n = 10): Objective tumor shrinkage, 5/10 (50%) Stable disease, 4/10 (40%) In most patients the tumor response was very durable http://blog.curemeso.org/breaking-news-data-anetumab-ravtansine-study/; Blumenschien GR et al. Proc ASCO 2016;Abstact 2509.
Phase I JAVELIN Trial Standard 3 + 3 dose escalation Dose expansion: Avelumab 10 mg/kg q2wk Mesothelioma (n = 53) NSCLC first line Ovarian cancer Colorectal cancer Ovarian platinum- refractory NSCLC second line Metastatic breast cancer Adrenocortical carcinoma Gastric/GEJ cancer first line-Mn/ second line Castration- resistant prostate cancer Urothelial cancer Melanoma Gastric/GEJ third line Urothelial post-platinum SCCHN post-platinum Renal cell carcinoma Mn = switch maintenance; SCCHN = squamous cell carcinoma of the head and neck ORR 5/53 (9.4%) Hassan R et al. Proc ASCO 2016;Abstract 8503.
Phase I JAVELIN Trial Results ORR (n = 53) = 5/53 (9.4%) For patients with 1% tumor cells (n = 39): PD-L1-positive, ORR = 2/20 (10.0%) PD-L1-negative, ORR = 2/19 (10.5%) Median duration of response was not reached Overall (n = 53) PD-L1-positive (n = 14) PD-L1-negative (n = 25) PFS Median PFS 17.1 weeks 17.1 weeks 7.4 weeks PFS at 24 weeks 38.4% 39.2% 40.7% Hassan R et al. Proc ASCO 2016;Abstract 8503.
Efficacy of Rovalpituzumab Tesirine in Recurrent or Refractory Small Cell Lung Cancer Outcome All patients 50% DLL3 expression ORR* (n = 60, 26) 18% 39% Median OS (n = 68, 29) 4.6 mo 5.8 mo One-year OS (n = 68, 29) 18% 32% ORR All patients 50% DLL3 expression As second-line therapy (n = 32, 14) 13% 29% As third-line therapy (n = 28, 12) 25% 50% *By investigator review Rudin CM et al. Proc ASCO 2016;Abstract LBA8505; Rudin CM et al. Lancet Oncol 2017;18:42-51.
Phase III ECOG-E1505 Trial: Safety and Efficacy by Cisplatin Chemotherapy Partner Squamous (n = 422) Nonsquamous (n = 1,078) Grade 3-5 toxicity Vino Doce Gem Vino Doce Gem Pemetrexed Anemia 12% 3% 15% 12% 3% 7% 4% Febrile neutropenia 6% 1% 7% 2% 0% 9% 15% Neutrophil count decrease 39% 41% 58% 40% 44% 12% 54% Platelet count decrease 3% 2% 3% 2% 1% 23% 12% Fatigue 15% 17% 12% 15% 13% 9% 9% Post-hoc nonrandomized subset analysis demonstrates no differences in OS or DFS for patients receiving all 4 adjuvant cisplatin-based chemotherapy regimens. Wakelee HA et al. Proc ASCO 2016;Abstract 8507.
Treatment of Oligometastatic NSCLC If a patient does present with oligometastatic disease, I think it s important to give them systemic therapy up front, just to make sure that there s no other disease lurking that you can t that you haven t identified. And if the patient does well there s not a significant tumor burden that s developing then I think consolidation with radiation therapy makes sense. I ve actually done this in small-cell lung cancer. George R Blumenschein Jr, MD
Case Discussion A 70-year-old man with a diagnosis of metastatic squamous cell lung cancer He completed 6 cycles of carboplatin/paclitaxel Repeat imaging about 2 months later revealed progressive disease What are his treatment options?
Phase III SQUIRE Trial of Gemcitabine/Cisplatin with or without Necitumumab for Stage IV Squamous NSCLC PR CR SD Necitumumab q3w Gemcitabine/cisplatin + necitumumab q3w PD 1 PD R Maximum of 6 cycles 1 Gemcitabine/cisplatin q3w PD Neci/Gem/Cis (n = 545) Gem/Cis (n = 548) p-value Primary endpoint HR Median overall survival 11.5 mo 9.9 mo 0.84 0.01 Thatcher N et al. Lancet Oncol 2015;16(7):763-74.
Phase III SQUIRE Trial of Gemcitabine/Cisplatin with or without Necitumumab for Stage IV Squamous NSCLC PR CR SD Necitumumab q3w Gemcitabine/cisplatin + necitumumab q3w PD 1 PD R Maximum of 6 cycles 1 Gemcitabine/cisplatin q3w PD More patients in the necitumumab arm had Grade 3 or 4 hypomagnesemia and Grade 3 rash Thatcher N et al. Lancet Oncol 2015;16(7):763-74.
Ongoing Lung-MAP (SWOG-S1400): Biomarker- Targeted Second-Line Therapy for Recurrent Stage IV Squamous Cell Lung Cancer NCT02154490 Common biomarker profiling Biomarker-driven substudies Nonmatch substudy S1400I Checkpoint na ve S1400B PI3K S1400C CCGA S1400D FGFR Nivolumab Taselisib Palbociclib AZD4547 Nivolumab + ipilimumab http://www.lung-map.org/about-lung-map; www.clinicaltrials.gov
Multiplex Testing in Squamous NSCLC Although I ve not seen it, there have been reports in the literature of patients having EGFR mutation although they have a squamous cell cancer. So I think there are probably mixed histologies out there that just weren t captured. So I think it s a reasonable thing to do We don t have an established biomarker that requires tissue testing in order to get a therapy. But I think in order to make that door open to patients, testing now is a way to go as these new drugs are coming along. George R Blumenschein Jr, MD
Gene Mutation Testing in Nonsquamous NSCLC All patients disease should be tested NGS would be a preferred approach Both serum and tissue should be tested if available In community practice, if the disease is ROS1- negative, ALK-negative and EGFR-negative, no drugs are approved for other targets However, data are emerging with RET and BRAF inhibitors So identifying those genes is important George R Blumenschein Jr, MD
Treatment Selection Algorithm for Patients with Disease Progression on an EGFR TKI and without T790M Mutations About 60% of patients with NSCLC harbor the T790M mutation. For the remaining 40% with T790M mutation-negative disease, we test for other targetable gene alterations, such as MET alteration, with known inhibitors or one that is being investigated on a clinical trial. If the patient does not fall under these categories, then it s appropriate at that point to initiate chemotherapy. The treatment selection depends on where the patient is in his or her treatment cycle. George R Blumenschein Jr, MD
Case Discussion A 70-year-old man presented in September 2014 with adenocarcinoma of the lung and liver metastases. Patient has left pleural nodularity. Patient received erlotinib until June 2016, when circulating cell-free DNA and repeat biopsy revealed the T790M mutation. He received osimertinib with response but has waxing and waning skin rash
Phase I/II Study of Olmutinib (BI 1482694) in T790M-Positive NSCLC Clinical variable, n (%) ORR Olmutinib (n = 70) 43 (61%) Disease control rate 63 (90%) Median duration of response 8.3 mo Most common drug-related adverse events (all grades, n = 76): Diarrhea 59% Pruritus 42% Rash 41% Nausea 39% Park K et al. Proc ASCO 2016;Abstract 9055.
Results from the Ongoing Phase I Trial of ASP8273 in EGFR Mutation-Positive NSCLC All patients Olmutinib (n = 63) ORR 19 (30%) Disease control rate 35 (56%) T790M mutation-positive subset (by local laboratory testing) n = 58 ORR 18 (31%) Disease control rate 33 (57%) Adverse events (n = 63): Hyponatremia: All grades, 19%; Grade 3, 13% Yu HA et al. Proc ASCO 2016;Abstract 9050.
Case Discussion A 53-year-old woman presents with KRAS mutation- positive lung adenocarcinoma and brain, lung and liver metastases. She receives 4 cycles of carboplatin/pemetrexed followed by docetaxel (3 cycles) followed by whole brain radiation therapy. She also received 19 cycles of nivolumab on a clinical trial
Case Discussion A 53-year-old woman presents with KRAS mutation- positive lung adenocarcinoma and brain, lung and liver metastases. She receives 4 cycles of carboplatin/pemetrexed followed by docetaxel (3 cycles) followed by whole brain radiation therapy. She also receives 19 cycles of nivolumab on a clinical trial. Upon disease progression she received 4 cycles of carboplatin/pemetrexed nivolumab rechallenge
Personal Approach to Immune Checkpoint Inhibitors for Patients with Preexisting Autoimmune Diseases My general approach has been not to enroll anybody who has an autoimmune illness We re starting to see some opening of or relaxing of those rules, and we re looking at patients and making determinations: Do they have to be on therapy for their autoimmune illness? Are they having symptoms from it? How severe is it? Those things are all getting factored in there. But in general, if someone has an autoimmune illness I don t offer them immunotherapy. I think the risk-benefit ratio is not there yet. George R Blumenschein Jr, MD
Case Discussion A 45-year-old man with ALK-positive adenocarcinoma of the lung and a single right frontal lobe brain metastasis Crizotinib therapy was initiated and a chest tube was placed to manage his empyema He is currently responding to crizotinib What are the therapeutic options if he experiences disease progression on crizotinib?
J-ALEX: Phase III Trial of Alectinib versus Crizotinib Eligibility Stage IIIB/IV or recurrent ALK-positive NSCLC Central testing for ALK rearrangement 1 prior chemo regimen 1 measurable lesion Treated/asymptomatic CNS metastases Alectinib 300 mg BID (PO) 28-day cycle R Crizotinib 250 mg BID (PO) 28-day cycle By independent review facility Median progression-free survival Alectinib (n = 103) Crizotinib (n = 104) p-value HR Not reached 10.2 mo 0.34 <0.0001 Objective response rate 91.6% 78.9% Nokihara H et al. Proc ASCO 2016;Abstract 9008.
