Manufacturing Innovations and Standards to Enable the Translations of Cellular and Gene Therapy Products
"This comprehensive overview delves into the advancements in manufacturing processes, standards, and collaborations driving the translation of cellular and gene therapy products. It discusses key developments in industry partnerships, tools, measurement sciences, and regulatory frameworks, emphasizing the critical role of innovative methodologies and reference materials. The narrative also highlights the pivotal contributions of organizations like NIST in facilitating the growth of the industry ecosystem and fostering global standards. Furthermore, insights into bioprocessing, analytical methods, and biobanking underscore the continuous evolution and standardization efforts within the biotechnology sector."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
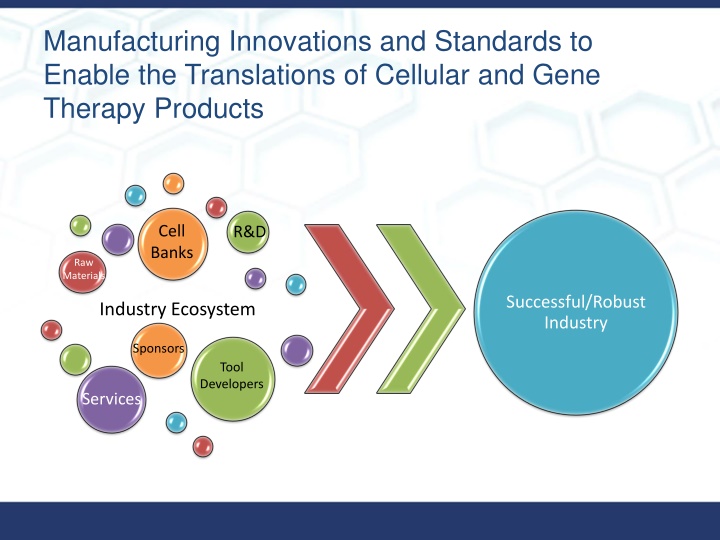
Manufacturing Innovations and Standards to Enable the Translations of Cellular and Gene Therapy Products Cell Banks R&D Raw Materials Successful/Robust Industry Industry Ecosystem Sponsors Tool Developers Services
AMTech on CMC Mforesight Open call NNMI MOU discussion SCB Strong interagency partnership Stakeholder engagement Convene workshops Bridging the Communication Gap Standards Leadership Innovative measurement sciences; enable tools, methods, reference materials, and data Increased industry collaborations NIST Laboratory Programs ISO/TC 276 leadership Convening of National and International meetings NIST laboratory programs underpin standards development
NIST Laboratory Programs: Measurement infrastructure for Cell and Gene Therapy and other Regenerative Medicine Products Bioinformatics and Modeling Tools Reference Materials Methods & Protocols Enabling Tools Cell Development of predictive models Imaging tools for biomarker validation Fluorescence benchmarking RM characterization (ID, quantity, function) Cell Therapy Flow cytometry RM Validation of gene editing tools Gene Therapy Whole genome RM Targeted NA quantification Bioinformatics pipelines Image analysis and computational tools Scaffold structure & properties Validated metrics of 3D scaffolds Tissue Engineering Reference scaffolds
ISO/TC 276: Biotechnology Secretariat: DIN WG3: Analytical Methods: Secretary: Lena Krieger Cell Counting (2 Parts) Characterization of Cells (3 Parts) Quality considerations for targeted nucleic acid quantification methods Methods to determine the concentration of total nucleic acids Methods to evaluate the quality of massive sequencing data Chairperson: Ricardo Gent 22 participating countries 13 observing countries Terms and Definition Biobanking and Bioresources WG4: Bioprocessing: Analytical Methods Ancillary materials present during the production of cells for therapeutic use (3 Parts) Downstream cell bioprocessing Cell transportation Bioprocessing Data Processing and Integration
The T-Cell Warriors Four years after a tentative but tantalizing breakthrough against leukemia, Carl June and Bruce Levine have gone from the fringes of gene therapy to the center of a revolutionary approach to cancer treatment. http://thepenngazette.com/the-t-cell- warriors/