Mastering Electrochemical Cells: Overview, Terminology, Calculations & Practical Applications
Explore the fundamentals of electrochemical cells, including voltaic and galvanic cells, cell diagrams, shorthand notations, and practical examples. Learn how to identify, draw, and understand the properties of saturated and unsaturated fatty acids. Enhance your knowledge of oxidation numbers, balance reactions, and label anodes/cathodes in galvanic cell configurations. Useful resources and cheat sheets are provided to aid in your learning journey.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
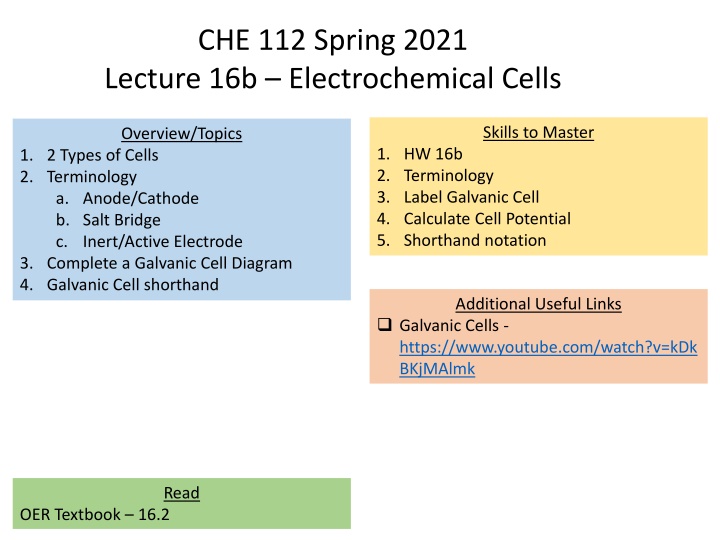
CHE 112 Spring 2021 Lecture 16b Electrochemical Cells Skills to Master Overview/Topics 1. HW 16b 2. Terminology 3. Label Galvanic Cell 4. Calculate Cell Potential 5. Shorthand notation 1. 2 Types of Cells 2. Terminology a. Anode/Cathode b. Salt Bridge c. Inert/Active Electrode 3. Complete a Galvanic Cell Diagram 4. Galvanic Cell shorthand Additional Useful Links Galvanic Cells - https://www.youtube.com/watch?v=kDk BKjMAlmk Read OER Textbook 16.2
Can you: Identify and/or Draw a Saturated or Unsaturated FA? Structure -> Physical Properties -> Biological Property Cheat Sheet: You will have Table 28.1 on all exams
Electrochemical Cells - Overview Voltaic Cells use an e- current to drive a nonspontaneous reaction (Alessandro Volta 1754-1827) Galvanic Cells use a spontaneous reaction to generate e- (Luigi Galvoni 1737-1798) Galvanic A + B C + D + electricity Voltaic
Cd (s) + Sn+2 (aq) Cd+2 (aq) + Sn (s) Galvanic Cell 1. Assign Oxidation Numbers 2. Balance the reactions 3. Label Anode and Cathode a. LEO (oxid anode) b. GER (red cathode) 4. Write reactions in proper boxes 5. Label the metal/solution 6. Direction of e- flow and # e- 7. Label the Salt Bridge 8. Miscellaneous Active Electrode conduct e- phase separated part of reaction Salt Bridge Maintains charge balance
Galvanic Cell - Answer 2 - + NO3- Na+ Cathode Anode (GER) Gain Mass Low E (LEO) Lose Mass High E 2 e- 2 e- Sn+2 (aq) Cd+2 (aq) Sn (s) Cd (s) Sn+2 (aq) + 2 e- Sn (s) Cd (s) Cd+2 (aq) + 2 e-
Galvanic Cell short hand notation Anode Cathode Cd (s) | Cd+2 (aq) || Sn+2 (aq) | Sn (s) Phase Boundary Salt Bridge Phase Boundary May or May not include concentrations Ex: Cd+2 (aq) (1.0 M)
Galvanic Cell Gases 1. Assign Oxidation Numbers 2. Balance the reactions 3. Label Anode and Cathode a. LEO (oxid anode) b. GER (red cathode) 4. Write reactions in proper boxes 5. Label the metal/solution 6. Direction of e- flow and # e- 7. Label the Salt Bridge 8. Miscellaneous You Try It! Complete the Diagram Write the Cell Notation Gas generally bubbled into the solution Inert Electrode conduct e- phase separated d/n react Given the reaction: Cr+1 (aq) + Cl2(g) Cr+3 (aq) + 2 Cl-1 (aq)
Cr+1 (aq) + Cl2(g) Cr+3 (aq) + 2 Cl-1 (aq) Galvanic Cell You try it!
Cr+1 (aq) + Cl2(g) Cr+3 (aq) + 2 Cl-1 (aq) 2 - + Na+ NO3- Anode Cathode (LEO) (GER) Cl2 (g) Cr+3 2e- 2e- Cr+1 2 Cl-1 (aq) Cl2 (g) + 2e- 2 Cl-1 (aq) Cr+1 (aq) Cr+3 (aq) + 2e-
Galvanic Cell short hand notation Anode Cathode Pt|Cr+1 (aq), Cr+3 (aq) || Cl2 (g), 2Cl- (aq) | Pt Phase Boundary Phase Boundary Salt Bridge






















