Mastering Electrode and Cell Potentials in Chemistry
Explore the fundamentals of cell potential and voltage, including Ecell determination, relationship with Gibbs free energy, and Nernst equation. Gain insights into spontaneous redox reactions, standard state conditions, and tabulated values. Understand the Diagonal Rule and standard reduction potentials for predicting reaction spontaneity.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
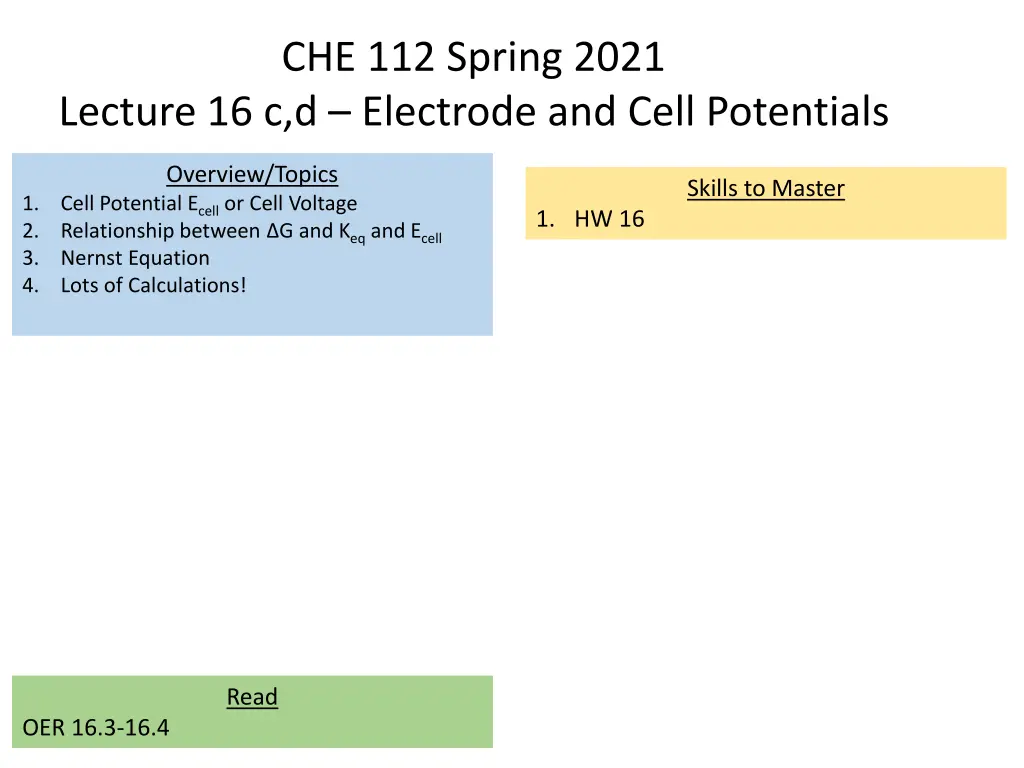
CHE 112 Spring 2021 Lecture 16 c,d Electrode and Cell Potentials Overview/Topics Skills to Master 1. 2. 3. 4. Cell Potential Ecell or Cell Voltage Relationship between G and Keq and Ecell Nernst Equation Lots of Calculations! 1. HW 16 Read OER 16.3-16.4
Cell Potential/Voltage - Basics Ecell(EMF) o Potential energy difference between cathode and anode responsible for the flow of electrons from the anode cathode o Determine spontaneity of redox reactions o Opposite Sign Convention for G o Units: Volt (V) Ecell = Ecathode - Eanode Ecell = Eox + Ered 1 J 1 C 1 V = Eanode o Other Units 1 A 1 sec 1 C = 1 J 1 sec 1 W = On Cheat Sheet Ecathode
Cell Potential/Voltage cont. o Opposite Sign Convention for G EMF > 0 (+) = spontaneous EMF = 0 = equilibrium EMF < 0 (-) = nonspontaneous o EMF Depends on: [R] and [P] Temperature Identity of Cation/Anion o E = standard state (STP) o STP = 25 C, 1 bar (~1 atm), 1 M solutions o Tabulated Values in Table 16.1 or Appendix L
Same concept as tables for H, G, S etc. Middle Appendix L Standard Reduction Potentials Top Table 6.1 o Can t tabulate every possible electrochemical cell o Tabulated values for cell reactions (written as reductions E red) o Zero arbitrary Standard Hydrogen Electrode (SHE) Bottom Ecell = Ecathode - Eanode Ecell = Eox + Ered
Top of Table Favored o Strongest Oxidizing Agent o Most reduced (lowest E element) o Good Cathodes (most + value) Miscellaneous o Diagonal Rule any species on the left will react spontaneously with a species that is lower/right. (Only applies Table 16.1) o More + reaction always reacts with a more negative reaction o Same as the Activity Series (1st semester, SD reactions). Bottom of Table o Strongest Reducing Agent o Most oxidized (highest E element) o Good Anodes (most value) Favored
Diagonal Rule o Diagonal Rule any species on the left will react spontaneously with a species that is lower/right. (Only applies Table 16.1) o More + reaction always reacts with a more negative reaction Note: Table 16.1 = in order high to low Appendix L = alphabetical order Example Will occur spontaneously F2(g) + 2 Li (s) 2 F- (aq) + 2 Li+ Example Will NOT occur spontaneously Sn+2 (aq) + 2 Ag (s) Sn (s) + 2 Ag+1 (aq)
Activity Series Standard Reduction Potentials
Lets Try It You have a Galvanic Cell with the following reaction: Cd (s) + Sn+2 (aq) Cd+2 (aq) + Sn (s) (a) Write the anode reaction (b) Write the cathode reaction (c) Calculate the Cell potential (d) Spont. or Nonspont.?
Lets Try It You have a Galvanic Cell with the following reaction: 2 Cr+3 + 3 I2 + 7 H2O Cr2O7-2 + 14 H+ 6 I- (a) Write the anode reaction (b) Write the cathode reaction (c) Calculate the Cell potential (d) Spont. or Nonspont.?
Problem Types 1. Solve for Ecell 2. Solve for Ecathode 3. Solve for Eanode 4. Spontaneous/Nonspontaneous 5. Given elements make a battery 6. Picture + any of above
On Cheat Sheet Relationship between Ecell G and Keq F = Faraday Constant charge on 1 mole of e- 96,500 C/mol e- 96,500 J/V mol RT nFlnKeq ? = ??????? Ecell = R = 8.31 J/K mol T = Temp (K) n = mols of e- - b/c sign conventions are opposite Michael Faraday (1971-1867) Developed foundations for understanding electricity
Relationship between EcellG and Keq (Ch. 12) Equilibrium (Kc) G = -RT ln Kc RT nFlnKeq Ecell = Kc= e G RT (Ch. 13) (Ch. 16) Thermodynamics ( G) Electrochemistry Ecell ? = ???????
Lets Try It! For the given reaction at 25 C, you solved for ????? = +0.2655 V Calculate (a) G (b) Keq (c) Gf (Sn+2) Cd (s) + Sn+2 (aq) Cd+2 (aq) + Sn (s)
Nernst Equation non-equilibrium o Concentration Cells o pH meters o Heartbeat o Standard State = 1.0 M or 1.0 atm and 298K G = G + RT lnQ o Just like for Gibbs Free Energy, Keq 0.0592 V RT nFlnQ Ecell= Ecell logQ Ecell= Ecell n Replace Constants, 298K switch to log Useful in pH problems
Given the following electrochemical cell at the given concentrations at 298 K, calculate Ecell. Is the reaction spontaneous as written? Lets Try It Note - solved previously reversed so now spontaneous Ecell = 0.6965V Cr2O7-2 + 14 H+ 6 I- 2 Cr+3 + 3 I2 + 7 H2O [2.0 M] [1.0 M] [1.0 M] [1.0x10-5 M] [(s) = ignore] [(l) = ignore]
Given a Zn-H+ cell with [Zn+2] = 1.0 M, P(H2) = 1.0 atm, Ecell = 0.45 V at 25 C Calculate the pH (ie [H+]) Lets Try It
A Concentration Cell is constructed according to the following picture. Calculate Ecell Lets Try It






















