Mastering Scientific Notation & Significant Figures
"Explore the significance and practicality of scientific notation and significant figures in simplifying the representation of large and small numbers. Learn the rules for converting numbers and determining significant figures for accurate calculations and measurements. Uncover the ease and precision scientific notation offers in scientific and mathematical contexts."
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Scientific Notation & Significant Figures
Scientific Notation Why do we need it? It makes it much easier to write extremely large or extremely small numbers.
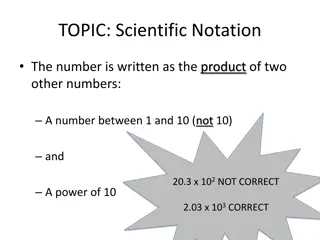
Scientific Notation What would you rather write? 602,200,000,000,000,000,000,000 Or 6.02 x 1023
Scientific Notation 6.02 x 1023 Writing the number like this means you will take the number 6.02 and multiply it by 10 twenty three times
Scientific Notation Every time the decimal moves left or right one place it is divided or multiplied by ten.
Scientific Notation 101= 10 103= 1,000 105= 100,000 10-1= 1 10-3=0.01 10-5= 0.00001
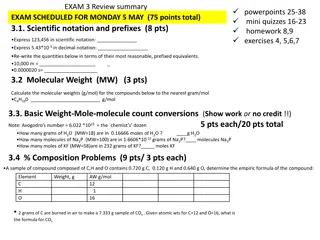
Scientific Notation 1. Move the decimal so that there is only one non zero number in front of it 5,050,000,000
Scientific Notation 1. Move the decimal so that there is only one non zero number in front of it 5,050,000,000 5.050000000
Scientific Notation 1. Move the decimal so that there is only one non zero number in front of it 2. Count how many places the decimal had to move 5.050000000 9
Scientific Notation Move the decimal so that there is only one non zero number in front of it Count how many places the decimal had to move This number now becomes the exponent in the scientific notation. If the decimal moved to the left the exponent is positive, if it moved to the right the exponent is negative. 5.05 x 109 1. 2. 3.
5,050,000,000 Equals 5.05 x 109 They are the same number
Try a really small number 0.0000000122 Write this number in scientific notation.
Try a really small number 0.0000000122 1.22
The decimal moved left how many spaces? 0.00000001.22 8 Write this number as the exponent. 1.22 x 10-8
Significant Figures
Sig Figs answer the question of How many places should I round to? or How many decimals do I keep?
Accuracy how close a measurement is to the true value of the quantity measured Precision the exactness of a measurement
The number of sig figs you keep in an answer totally depends on the precision and accuracy of the instruments you are using.
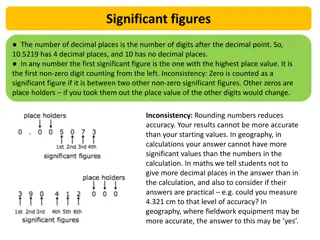
Rules to find Sig Figs 1.If the Number is not a zero it is significant 2.If zeros are in between two non zero numbers it is significant 3.Zeros before a non zero number are not significant. 4. Zeros after non zero numbers are significant only if there is a decimal.
352 4505 0.00324 1240 130.0
When Multiplying/Dividing keep the smallest number of sig figs in the answer. When Adding/Subtracting keep the smallest number of decimals in the answer.