Metal-Metal Bonding in Inorganic Chemistry
Understanding the complexities of metal-metal bonding in inorganic chemistry, including covalent and dative bonding types, various electron counts, symmetry interactions, and examples of multiple bonded compounds such as double and triple bonds.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
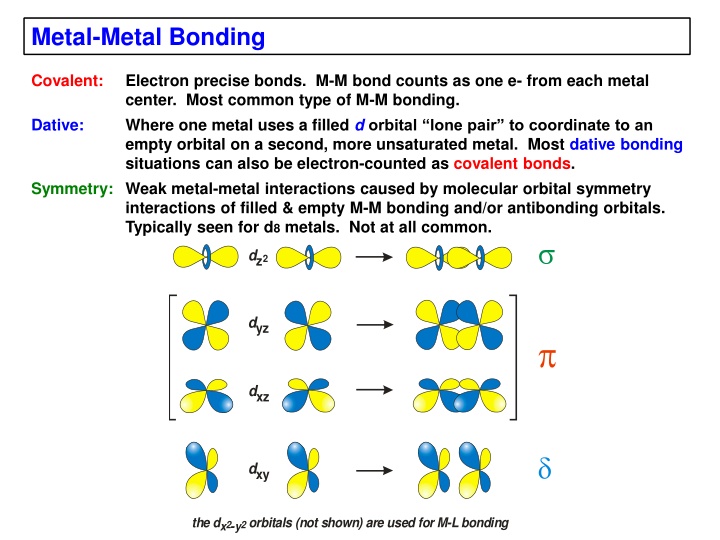
Metal-Metal Bonding Covalent: Electron precise bonds. M-M bond counts as one e- from each metal center. Most common type of M-M bonding. Where one metal uses a filled dorbital lone pair to coordinate to an empty orbital on a second, more unsaturated metal. Most dative bonding situations can also be electron-counted as covalent bonds. Symmetry: Weak metal-metal interactions caused by molecular orbital symmetry interactions of filled & empty M-M bonding and/or antibonding orbitals. Typically seen for d8 metals. Not at all common. Dative: dz2 dyz dxz dxy the d- orbitals (not shown) are used for M-L bonding x y 2 2
L M L L L L M-M antibonding orbitals M L L L dxy dxy dyz dxz dz2 dyz dxz dz2 M-M bonding orbitals the d - , s and p orbitals are not shown since they are used for M-ligand bonding 2 2 x y x,y Electron Count d1- d1 d2- d2 d3- d3 d4- d4 d5- d5 d6- d6 d7- d7 d8- d8 Resulting M-M Bond Single bond Double bond Triple bond Quadruple bond optimum Triple bond Double bond (M-L bonding usually dominates) Single bond No bond (symmetry interaction)
Some Covalent Multiple Bonded Examples: Double Bonds R t-Bu t-Bu O O R Cl Cl Cl Cl O O Ta Ta Cl Os Os Cl O R O O O Cl Cl O O R Ta=Ta = 2.68 Os=Os = 2.30 Triple Bonds d5-d5Triple Bond Chisholm d3-d3Triple Bonds O O PhH2C PhH2C C C CH2Ph Cr Cr Mo Mo CH2Ph C C O PhH2C O CH2Ph Mo-Mo = 2.17 Cr-Cr = 2.27
Quadruple Bonds (Cotton) d4-d4 electronic configurations often lead to the formation of quadruple M-M bonds. Prof. F. Albert Cotton at Texas A&M was famous for his discovery and extensive studies of M-M quadruple bonds (and other M-M bonded systems). 2- H3C CH3 Re H3C CH3 H3C CH3 Re H3C CH3 F. Albert Cotton Texas A&M University Re-Re = 2.18 Me Me O O 4 Cr Cr Cr-Cr = 1.85
Dative M-M Bonds (unsymmetrical M-M bonded complexes) t-Bu t-Bu Ni-P = 2.16 Ni-P = 2.24 P tetrahedral coordination like Ni(0) planar coordination like Ni(+2) O O C O C Ni Ni C P Ni-CO = 1.70 Ni-CO = 1.78 t-Bu t-Bu Ni-Ni = 2.41 Covalent M-M Bonding Dative Left Ni Ni(+1) [ -PR2] -PR2 CO M-M Right Ni Ni(+1) [ -PR2] -PR2 2CO M-M Left Ni Ni(+2) 2[ -PR2] 4e- CO Ni Ni(0) 2e- Right Ni Ni(0) 2 -PR2 2CO d9 2e- 2e- 2e- 1e- d9 2e- 2e- 4e- 1e- d8 d10 4e- 4e- 2e- Total 16e- Total 18e- Total 16e- Total 18e-
Problem: Electron-count the following complex using both the covalent and dative M-M bonding methods: R2 P Me3P Me3P CO CO W Re CO Me3P CO CO OC Problem: Electron-count the following complex. What is the order of the Re- Re bond? Why wouldn t it be appropriate to use the dative bond method for this complex? PR3 Cl Cl Re R3P R3P Cl Re Cl PR3
Weak M-M Interactions by Symmetry Based on the MO diagram at the beginning of this section, d8-d8systems shouldn t have any M-M bonding due to the filling of all the M-M antibonding orbitals, which cancels out the M-M bonding orbitals. But Harry Gray and others noted that more than a few bi- or polymetallic d8 complexes do show the presence of weak M-M bonding interactions, both in solution and the solid-state. Harry Gray Caltech L L NR C L L RNC N R CNR Ir M M L L C L L Cl R3P Ir pz pz C C N NR R NR C RNC N R CNR Ir dz2 dz2 C