Metallocenes: Structural Features and Properties
Explore the history of cyclopentadienyl ligands, the synthesis and properties of ferrocene, and the structural features of metallocenes. Learn about the reactivity of cyclopentadiene, the Nobel Prize-winning discovery of ferrocene, and the unique characteristics of various metallocene complexes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
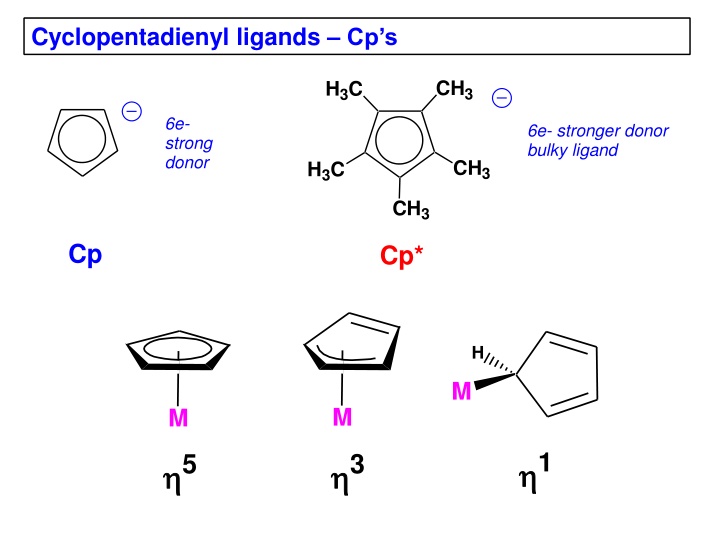
Cyclopentadienyl ligands Cps CH3 H3C 6e- strong donor 6e- stronger donor bulky ligand CH3 H3C CH3 Cp Cp* H M M M 1 5 3
Free neutral cyclopentadiene, which is deprotonated with a strong base to generate the Cp , is unstable and reacts with itself via a Diels-Alder reaction to make the dicyclopentadiene. One typically regenerates cyclopentadiene by distilling ( cracking ) it from the high boiling dimer solution and storing it in a refrigerator, but it slowly re-dimerizes to make dicyclopentadiene. +
Brief History of Ferrocene: 1901 Synthesis of KC5H5 from K and C5H6 Miller, Tebboth & Tremaine Sythesis of Fe(C5H5)2 from the reaction of C5H6 with freshly reduced Fe at 300 C 1951 Kealy & Pauson 3C5H5MgBr + FeCl3 Cp2Fe + fulvalene + 3MgBrCl They were trying to make fulvalene! They proposed that they had made: 1951 Fe E. O. Fischer proposes a Double-cone structure X-ray structural data Diamagnetism Chemical behavior 1952 Geoffrey Wilkinson & Robert Woodward: Sandwich Structure IR spectroscopy Diamagnetism Dipole moment = 0 1952 Woodward noted that the Cp rings were susceptible towards electrophillic substitutions, similar to the aromatic behavior of benzene. Thus the common name: ferrocene Fischer & Wilkinson receive the Nobel Prize in Chemistry for their discovery of ferrocene, which played a key role in opening up the new area of organometallic chemistry. 1973
Some Properties of Metallocenes Complex Color mp/ C 200 (decomp.) Miscellaneous bimetallic with two -H bridges and a fulvalene bridging ligand (structure shown later) Ti(C5H5)2 green purple 167 very air-sensitive, paramagnetic bimetallic with 1, 5-C5H4 bridges and terminal hydrides (structure shown later). very air-sensitive several bimetallic isomers with fulvalene and 1, 5 bridges and terminal hydrides (structures shown later), diamagnetic, air- sensitive. same as Mo air-sensitive and easily hydrolyzed, interesting high-spin to low-spin interconversion air-stable, can be oxidized to blue-green [Fe(C5H5)2]+which, in turn, is a good inert oxidizing agent. V(C5H5)2 Nb(C5H5)2 yellow - scarlet 173 Cr(C5H5)2 Mo(C5H5)2 Black - W(C5H5)2 yellow-green - brown 173 Mn(C5H5)2 orange 173 Fe(C5H5)2 air-sensitive, paramagnetic 19e- complex, can be oxidized to the air-stable 18e- yellow [Co(C5H5)2]+ 20e- complex, slow oxidation in air to the labile, orange cation [Ni(C5H5)2]+ purple-black 174 Co(C5H5)2 green 173 Ni(C5H5)2
Structural Features M M-C C-C Cp Cp Fe [Fe]+ 2.04 3.29 1.42 2.07 3.40 1.40 M-C Cp Cp M Ru 2.19 3.64 1.43 Os 2.19 3.61 1.45 Co [Co]+ 2.10 3.44 1.41 C-C 2.03 3.24 1.42 Ni 2.18 3.63 1.41 The changes in the neutral Fe, Co, Ni metallocenes are a direct result of going from 18e- (Fe) to 19e- (Co) to 20e- (Ni) counts. The extra electrons for the Co and Ni complexes are going into M-Cp antibonding orbitals, which are delocalized and progressively weaken the M-Cp bonding, leading to the increase in bond distances. This in spite of the fact that the metal s covalent radius is decreasing as one goes from Fe to Ni. Problem: Explain why the Fe-C distance lengthens for [Cp2Fe]+, while the Co-C distance shortens for [Cp2Co]+.
Oxidation of Cp2Os does not produce a simple cationic monomer as seen for Co and Fe. Instead one gets dimerization to produce the following bimetallic complex that has an Os-Os bond (3.04 ). Problem: Electron-count this complex. Is it para- or diamagnetic?
Bis-Cp Early TM Complexes The simple neutral bis-Cp complexes of the early transition metals are quite different because they are in very low +2 oxidation states (very electron-rich) and quite unsaturated. Thus, they are very reactive towards C-H oxidative additions and other reactions. Me Me Me Me Me CH2 Me Me Me Me H Ti Ti Ti Ti H Me Me H Me Me Me Me Me Me Me Me Problem: Electron-count this Ti2 complex. Is it para- or diamagnetic? H Nb Nb H
Mo-Mo = 3.19 Mo Mo THF H 50 C H Mo Mo H2O 100 C H h H Mo-Mo = 3.36 toluene 70 C Mo Mo H
Cp Variants these have special bonding properties important in substitution reactions (see that chapter) 2- indenyl (-) fluorenyl (-) fulvalenediyl (2-) 6e- donor Mo 2.45 azulene Behrens, Angew. Chem., 1987
MO Comparison of Cp vs. Arene Ligands Benzene-Metal Complex Cyclopentadienyl-Metal Complex metal d orbitals metal d orbitals M M