Methods for Concentration Expression in Solutions
Learn about different methods like formality, morality, normality, percent concentration, parts per million, and specific examples for preparing solutions in lab experiments.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
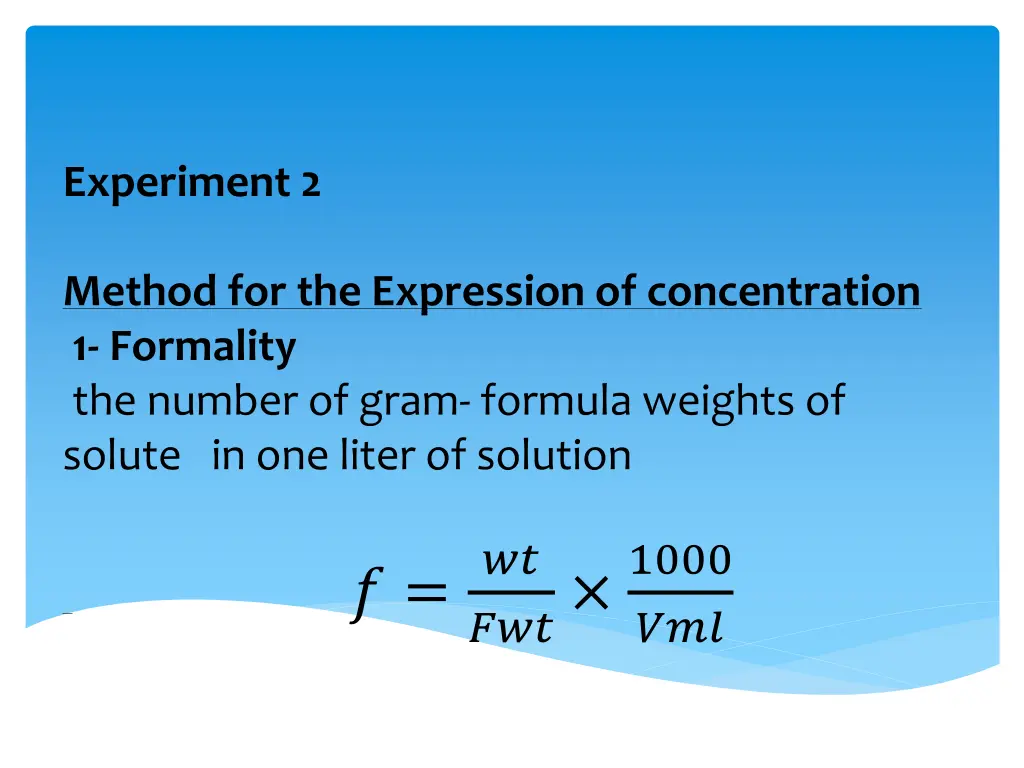
Experiment 2 Method for the Expression of concentration 1- Formality the number of gram- formula weights of solute in one liter of solution ?? ??? 1000 ? = ???
2- Morality the number of gram- molecular weights (or moles) in one liter of solution ?.?? 1000 ?? ? = ??? 3-Normality: the number of gram- equivalent weight in one liter of the solution ? = ??.?? 1000 ?? ???
Percent Concentration ???? ? ?? ?????? ???? ? ?? ???????? 100 Weight percent= ?????? ?? ?????? ?????? ?? ???????? 100 Volume percent = ???? ? ?? ??????.? ?????? ?? ???????? ?? 100 Weigh- Volume percent=
Parts per million ???? ? ?? ?????? ???? ? ?? ???????? 1.000.000 PPm= The Dilution before dilution = after dilution C1V1 = C2V 2
1-Solids a- Preparation (0.1N)of NaOH in250 ml ?? .???1000 ?? N = ??? V ml = 250 Eq. wt = Wt = X
2 Liquids b-Preparation of (0.1N) HCL from concentrated HCL ??? 1000 N(HCL ) = ??????????????? % ?? .??????? Specific gravity = 1.18 Eq.wt of HCL = 36.5 38 % ? ? of HCL is obtained from the reagent bottle ... N concentrated HCL = 12
To calculate the volume of concentrated HCL that should be taken to prepare 250 ml of (0.1N) HCL . Conc.HCL = N1 V1 12 X V1 V1 = ? diluted HCL = N2 V2 = 0.1 X 250






















