Microbiology and Treatment of Sewage
Domestic sewage contains a high concentration of microorganisms, with bacterial counts ranging from 50,000 to 5,000,000/ml. The process of bacterial reproduction in sewage, illustrated in the images, progresses through phases such as log phase, declining growth, stationary phase, increasing death phase, log death phase, and ultimately, depletion leading to bacterial death. This decomposition process is crucial for sewage treatment, as indicated by Biological Oxygen Demand (BOD) and Chemical Oxygen Demand (COD) measurements reflecting the biodegradability and oxidizability of pollutants. Understanding the microbial dynamics in sewage is essential for effective treatment methods to safeguard environmental and public health.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
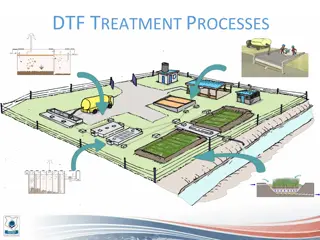
Microbiology of sewage and sewage treatment
Domestic sewage contains enormous quantities of microorganism. Bacteria counts in raw sewage may be expected to range from 50 000 to 5 000 000 / ml . In the presence od adequate food and oxygen, bacteria will reproduce as illustrated in the figure below
Log phase: it represent the time required for the organism to acclimate to their new environment Log phase: this phase coincides with the max rate of substrate removal.
Declining growth: this phase represents decreasing of growth phase Stationary phase: here the population remains stationary because the growth of new cells is offset by the death of od cells.
Increasing death phase; in this phase the death rate of bacteria begins to increase Log death phase : in this phase the death rate of bacteria exceeds the production of new cells Death: in this phase the substrate is depleted and the bacteria die due to lack in substrate.
BIOLOGICAL OXYGEN DEMAND Biological Oxygen Demand is a measure of the content of biologically degradable substances in the sewage water. The substances are broken down by micro-organisms in the presence of (and therefore with the consumption of) oxygen. Oxygen demand is measured in terms of the quantity of oxygen consumed by the micro-organism over a period of five days (BOD5) or seven days (BOD7) in decomposing the organic pollutants in the water at a temperature of 20oC.
CHEMICAL OXYGEN DEMAND Chemical Oxygen Demand indicates the quantity of the pollutants in the water that can be oxidised by a chemical oxidant.
TOTAL ORGANIC CARBON Total Organic Carbon is another measure of the quantity of organic materials, determined by measuring the quantity of carbon dioxide produced from combustion of a sample.
Calcining loss is obtained by first determining the dry solids content in a sample, and the calcining is so that the organic substance is burnt. The difference in weight before and after calcining represents the quantity of combustible substances and is a measure of the quantity of organic substance.
CARBON DIOXIDE The high solubility of CO2 reduces the pH of the water, which causes excessive consumption of lime or other neutralization agents in coagulation and softening processes. The corrosiveness of the water is also higher at lower pH values.
Exposure of water droplets to air for 2 s will lower the concentration of CO2 by 70 to 90%. Carbon dioxide can also be removed (converted into bicarbonates) by the addition of lime. It is recommended that aeration be used if CO2 concentrations are greater than 10 mg/L; otherwise lime addition should be used to neutralize the CO2 (AWWA, 1971). The economics of each process must be carefully compared at expected concentrations to select the appropriate process.
CORROSIVENES S The addition of oxygen to water makes the water more corrosive. As noted previously, the removal of CO2 will have the beneficial effect of raising the pH.
HYDROGEN SULFIDE The solubility of H2S is also very high because of its ability to react with water and form the ions HS- and S2-. Sulfides cause unpleasant tastes and noxious odors in a water. At high concentrations, H2S can be lethal. Removal of H2S is not as rapid as removal of CO2, and as CO2 is removed the pH rises, which causes the increased formation of the ionized forms of sulfides. These ionized forms are not removed by aeration. However, concentration of H2S is essentially zero, which enhances its rate of mass transfer. The standard (in Canada) for total sulfide concentration in drinking water is 0.05 mg/L. the atmospheric
IRON AND MANGANESE Iron and manganese are dissolved by groundwaters that have a high dissolved CO2 content and have a low pH. The more soluble forms of iron and manganese are their lower oxidation states, Fe (II) and Mn (II). The addition of oxygen by aeration promotes the oxidation of iron and manganese to their relatively insoluble forms, Fe (III) and Mn (IV). The precipitates formed will be removed in coagulation - sedimentation and filtration operations later on in the treatment train.
IRON AND MANGANESE The addition of oxygen to water makes the water more corrosive. As noted previously, the removal of CO2 will have the beneficial effect of raising the pH.
METHANE Methane, or marsh gas as it is commonly known, may be present in some waters at significant concentrations. It is very insoluble in water and its presence creates an explosion hazard.
ORGANIC MATTER The oxidation of organic matter and other inorganic substances causing oxygen demand will be accomplished to only a very limited extent by the addition of oxygen through aeration. This is not a reason to design an aeration system.
OXYGEN Oxygen will be transferred to waters below saturation concentration with effects described under various other categories.
TASTE AND ODOR Taste and odor causing compounds are often volatile and these are removed to a significant extent by aeration. Also, the addition of oxygen will improve the taste of a water to a limited extent.
VOLATILE ORGANICS Trace volatile organics that may be present in surface and groundwaters are stripped from the water by aeration. The degree of aeration required depends on the concentration and solubility of the compounds of interest. Stripping volatile toxic substances from a water or wastewater transfers the problem to the atmosphere. There is movement to require passing the exhaust gases from the stripper through activated carbon or other treatments to remove the contaminants. Using an air stripper with activated carbon treatment of the exhaust gas may be more economical than treating the water directly with activated carbon, which more rapidly exhausts.