Mole Calculations and Basic Triangle Problems
This content covers mole calculations including converting between moles, mass, and molecules for compounds like C6H12O6. It also delves into basic triangle problems and team efforts in solving them. The practical examples provided help in enhancing understanding of these fundamental chemistry concepts.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Keep Reading Chapter 5 Homework 5 due today, Friday 20 October
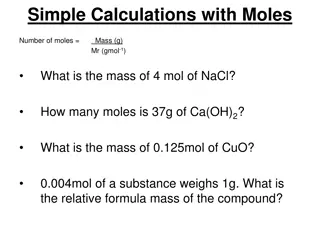
Multiply Down Moles (n) 6.0*1023 MW ALL ROADS LEAD THROUGH MOLES Weight (w) molecule count (N) Divide up
DIVIDE UP MULTIPLY DOWN
Sweet mole calculations part 1 a) weight per mole (MW): what does a mole of C6H12O6 weigh ? (C=12; H=1; O=16) 6*12 + 12*1 + 6*16 = 180 g/mol 1.1) mole to mass: how many g C6 H12O6 of are present in 0.00555 mole of C6 H12O6? 0.00555 mol * 180 g/mol = 1 g 1.2) mass to mole: how many moles of C6 H12O6 are in 360 g of C6H12O6? 360 g/180 g mol-1 = 2 moles
mole calculations part 1 (continued) 1.3) mole to # molecule: how many molecules of C6 H12O6 are in 0.5 mol of C6H12O6 ? 0.5 mol *6*1023 molecules/mol = 3*1023 molecules 1.4) mass to # molecules: how many molecules of C6 H12O6 are in 120 g of C6H12O6 ? (120/180) mol * 6*1023 molecules =4*1023 molecules mol
mole calculations part 1 (continued) 1.5) # molecules to moles: how many moles in 3*1024 molecules of C6 H12O6 ? 3*1024 molecules/ 6*1023 molecules/mol = 5 moles 1.6) # molecules to mass : How many grams of C6 H12O6 are in 2.0 *1022 molecules of C6H12O6 ? 2*1022 molecules * 180 g 6*1023 molecules/mol mol =0.0333 * 180 = 6 grams
BasicTriangle problems with clickers and team effort
How many grams of oxycontin (oxy), MW=315 g/mol are in 0.06349 mol of oxy ? A. 3.8*1023 g B. 4961 g C. 2.0 g D. 0.0002 g 25% 25% 25% 25% 2.0 g 4961 g 0.0002 g 3.8*1023 g
How many moles of CO, MW=28 g, are in 3*1023 molecules of CO ? Remember that 1 mol count=6*1023 A.2 B. 0.5 C. 8.4*1023 D.1.07 25% 25% 25% 25% 2 0.5 1.07 8.4*1023
How many molecules of heroin, MW=369 g/mol are in 61.5 g of heroin ? Remember that 1 mol count=6*1023 A.1*1023 B. 0.1667 C. 2.27*104 D.3.7*1025 25% 25% 25% 25% 0.1667 1*1023 2.27*104 3.7*1025
How many molecules of cocaine, MW=303.4 g/mol are in 10.11 g of cocaine? Remember that 1 mol count = 6*1023 . 25% 25% 25% 25% A. 1.8*1026 molecules B. 2*1023 molecules C. 2*1022 molecules D. 1.98*1021 molecules 2*1023 molecules 2*1022 molecules 1.98*1021 molecules 1.8*1026 molecules
Harder problems.team efforts with clickers
The gram molecular mass of of The gram molecular mass of of crystal meth many moles of crystal meth are in a typical street `teenth = 1/16 of many moles of crystal meth are in a typical street `teenth = 1/16 of an ounce= 1.80 grams ? an ounce= 1.80 grams ? ( (1 mol count=6.0*10 1 mol count=6.0*1023 crystal meth ) ) is is 149.2 g/mol. 149.2 g/mol. How How 23 .) .) 94% A. 7.27*1021 B. 0.0121 C. 82.9 D. 2.48*10-22 E. My answer isn t above 6% 0% 0% 0% 82.9 0.0121 7.27*1021 2.48*10-22 My answer isn t above
B1 B1- -Aflatoxin (MW=328.2 g/mol) Aflatoxin (MW=328.2 g/mol) is a naturally occurring, horribly toxic poison is a naturally occurring, horribly toxic poison derived from Aspergillus fungi, a common contaminant in corn. It causes cancer in derived from Aspergillus fungi, a common contaminant in corn. It causes cancer in most humans at the staggeringly low exposure level of 1*10 most humans at the staggeringly low exposure level of 1*1017 the equivalent mass of aflatoxin represented by this count ? the equivalent mass of aflatoxin represented by this count ? ( ( 1 mol count=6.0*10 count=6.0*1023 17 molecules. What is molecules. What is 1 mol 23 .) 58% A. 3.3*10-8 g B. 1.8*10-4 g C. 5.47*10-5g D. 5.06*10-10g E. Not any of above 26% 16% 0% 0% 5.47*10-5g 5.06*10-10g 3.3*10-8 g 1.8*10-4 g Not any of above
Micro chip manufacture requires nearly oxygen Micro chip manufacture requires nearly oxygen- -free conditions wherein the concentration of wherein the concentration of O O2 2 is at or below 17 pg/L. Given that the atomic mass of that the atomic mass of O O is is 16 16 g/mol, g/mol, about how many molecules of molecules of O O2 2 /L /L does this represent ? does this represent ? Note: 1 mol count=6.0*10 count=6.0*1023 free conditions is at or below 17 pg/L. Given about how many Note: 1 mol 23 A. 5.4*1024 B. 3.5*1022 C. 3.2*1011 D. 5.3*1022 94% 6% 0% 0% 5.4*1024 3.5*1022 3.2*1011 5.3*1022
Moles: part 2 Moles: part 2 body parts (mole ratio) math body parts (mole ratio) math: the knee bone is connected to the thigh bone .
How many hands ??? 1 6 5 2 3 4 7 9 8 1 0 11 = 22 hands 2 hands 1 people x 11 people
How many toes connected to the hands (assuming no deformities) ? 5 hands 1 person hand x 5 4 = 50 toes x 10 toes person 3 2 1
Simplest `body parts example : mole Octane = C8H18 How many H are in 20 moles of octane ? Mole 20 mols octane * 18 mol H = 360 mol H mol octane
Isohexane has the formula C6H14. How many C moles are present if we have 0.1667 moles of isohexane ? A. 0.0277 mol C B. 1.00 mol C C. 36 mol C D. 2.333 mol E. None of the above 0% 0% 0% 0% 0% 36 mol C 1.00 mol C 2.333 mol 0.0277 mol C None of the above
`body parts example 2 : Weight moles first, then mol Octane = C8H18 How many moles of octane contain 24 grams of C (at. wt. = 12 g/mol) Step 1) Convert grams C to moles C (all roads lead through moles divide up) Mol 24 g 12 g/mol C = 2 moles C
`body parts example 2 : (continued) Octane = C8H18 How many moles of octane contain 24 grams of C (at. wt. = 12 g/mol) Step 2) 2 moles C => how many moles octane? 1 mol octane = x 8 mol C 2 (body parts relationship ?) x= 2/8=0.25 moles octane
Isohexane has the formula C6H14. How many moles of isohexane are formed with 24 grams of C (1 mol C=12 g) A. 0.333 moles isohexane B. 2 moles isohexane C. 0.1667 moles isohexane D. 3 moles isohexane E. Stoichiometry blows F. None of the above 0% 0% 0% 0% 0% 0% 2 moles isohexane 3 moles isohexane 0.333 moles isohexane 0.1667 moles isohexane Stoichiometry blows F. None of the above
`body parts example 3 : Weight moles , then mol Octane = C8H18 (MW=114) How many grams of H are in 10 grams of Octane ? (1 mole H=1 g H) Step 1) Convert grams octane to moles octane All roads lead through moles; divide up Mol weight 10 g 114 g/mol = 0.0877 mol octane