Mole Calculations and Molecular Mass Formulas
Explore mole calculations, molecular mass concepts, and conversion methods for chemistry problems. Learn to calculate the molecular masses of compounds like H2O2 and Ca(NO3)2 and understand how to convert moles to molecules using the triangle method.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
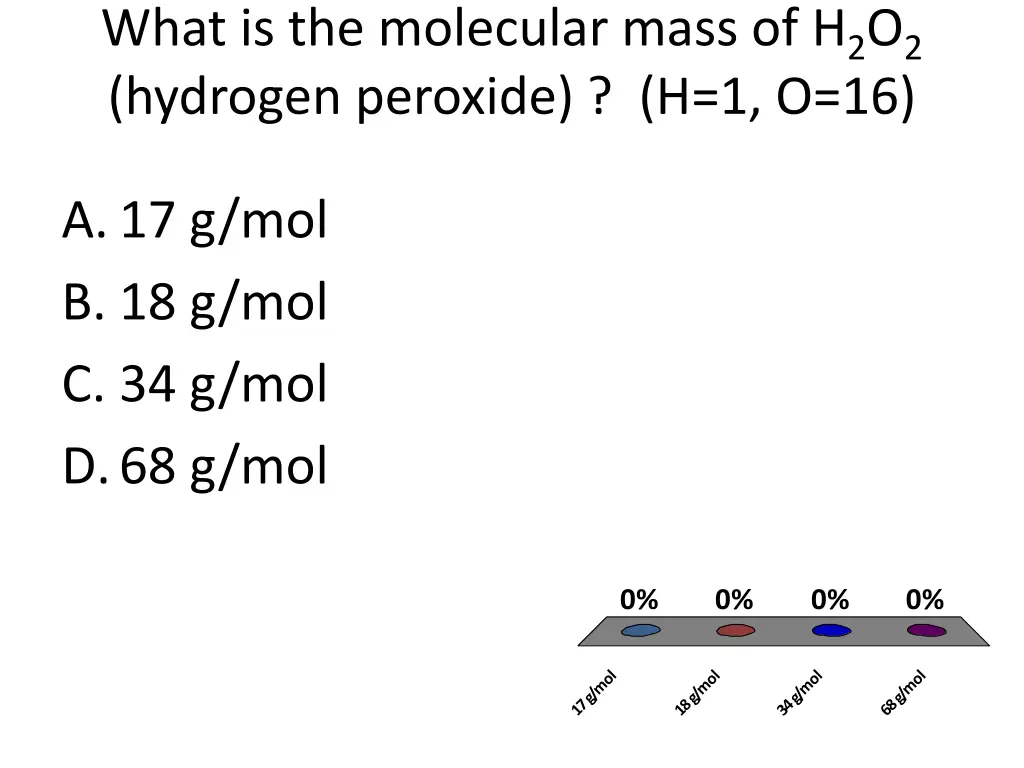
What is the molecular mass of H2O2 (hydrogen peroxide) ? (H=1, O=16) A.17 g/mol B. 18 g/mol C. 34 g/mol D.68 g/mol 0% 0% 0% 0% 17 g/mol 18 g/mol 34 g/mol 68 g/mol
What is the molecular mass of Ca(NO3)2(Ca=40, N=14, O=16) A.102 B. 164 C. 328 D.142 0% 0% 0% 0% 102 164 328 142
IN-CLASS simple mole calculations (on board) the dozen method way (continued)
The triangle `trick for those allergic to eggs Multiply Down Moles (n) 6.0*1023 MW ALL ROADS LEAD THROUGH MOLES Weight (w) molecule count (N) Divide up
Multiply down moles MW (g/mol) ~6.0*1023 # atoms or molecules weight (g) Divide up
Multiply down 0.5 Moles SiO2 MW SiO2 =60 weight (g) SiO2?? 0.5 *60= 30 g
Multiply down 5 Moles H2O 1 mol~6.0*1023 molecules # molecules of H2O ? 5*6*1023=30*1023 =3*1024
500 /100 = 5 moles CaCO3 Moles CaCO3??? MW CaCO3 (g/mol)=100 500 g CaCO3 Divide up
3*1025/6*1023=0.5*102=50 mol CO2 Moles of CO2 ?? 1 mol~ 6.0*1023 molecules 3*1025 molecules CO2 Divide up
MassMoles # molecules: around the triangle approach How many H2O molecules in 7200 grams of H2O ? 400 moles 7200 18 Divide up to moles Multiply down to molecules x 6.022*1023 400 x 6.022*1023 MW 18 g/mol Fill in MW weight # molecules 7200 g Start here =2.409*1026
In-class board mol- weight-count conversions using triangle method