Mole Conversions Guide
Discover the world of mole conversions through step-by-step activities and practical examples. Learn about converting between moles, grams, liters, molecules, and atoms using the provided drill, roadmap, and guided activities. Explore the concept of Standard Temperature and Pressure (STP) and enhance your understanding with engaging tasks. Start your journey in mastering mole conversions with this comprehensive guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
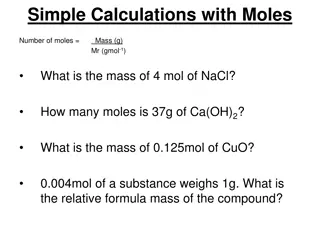
1 and 2 Step Mole Conversions
Drill Create your placemat on your giant whiteboard. Solve: How many moles are in 4.32 x 1024 molecules of H2O? **Need calculator and Periodic Table today!
Remember In Answer Number Units Substance Correct # of Sig Figs In Set-Up Number Units Substance
Agenda Drill Mole Conversion Chart Guided Activity Task Cards Exit Slip
I Can Convert between units of moles and units of grams, liters, molecules, and atoms Convert between units of grams, liters, molecules, and atoms
Mole Conversion Roadmap Take a minute What do you notice about this chart?
Chart What does STP mean? What are the units associated with it?
STP Standard Temperature and Pressure Temperature 0oC 273.15 K Pressure 1 atm
Mole Conversions Guided Activity Read the packet to solve 1 and 2 step problems You will use cards to put in the correct spot after making a grid on your giant whiteboard
When you are done with the Packet Check your answers to make sure they are correct and have all the parts you need Start the Task cards in your bin
Why Do We Do This?? 2NaCl 2Na + Cl2 If I have 23g of chlorine, how many grams of NaCl can I make?
Practice How many grams are in 2.600 moles of NaCl?
How many Liters are in 5.25 x 1024molecules of H2?