Molecular Spectra: Types and Bonds Explored
Explore the world of molecular spectra, including electronic, rotational, and vibrational-rotational spectra. Dive into molecular bonding concepts such as covalent and ionic bonds, and understand the role of vibration and rotation in spectral analysis. Discover the significance of energy levels, tunnelling probabilities, and the Frank-Condon principle in molecular spectroscopy.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
MOLECULAR SPECTRA MOLECULAR SPECTRA
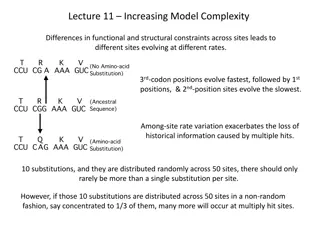
INTRODUCTION: The molecular spectra may be broadly divided into three types. MOLECULER SPECTRA MOLECULER SPECTRA Electronic spectra Pure rotational Vibrational- rotational
1)Molecular bond: Molecule is a stable system where in two or more atoms are bound together . A system can be stable if it possesses minimum energy. 2)Co-valent bond: A bond formed by sharing electron is called covalent bond . Eg.H2molecule 3)Ionic bond: If one or more electrons from one atom are transferred to other atom,then positive and negative ions are formed which strongly attract each other to form a stable system i.e.molecule.The bonding so formed is called ionic bond.
The simplest molecule H2in which two proton share an electron. Twoprotons form two boxes with wall in between them.
The probability of tunnelling decrease with increase in distance between the two protons
The rotation about the axis of symmetry involves energy about a few ev, which is of the order of bond energy.
Vibrational spectra Vibrational energy levels are equispaced . Ev (v+1/2) Pure vibrational spectra are observed only in liquid.
Vibration-Rotation spectra The spectra obtained by a low resolving power spectrometer will appear as a broad band called vibration-rotation band.
If a group of bands corresponds to same electronic transition,then they are called a system of bands. (i)If V=(V -V ) is a constant,then a series of such band is called a sequence. (ii)A set of bands corresponding to same v and different values of v or same value of v and different values of v is called a progression.
Frank-Condon principle and intensity of band spectra (i)The transitions should be represented by vertical lines (ii)The observed transitions should start from extreme positions of vibrational levels.