Neurosurgery Spine Conference at Stanford Hospital and Clinics
Comprehensive case presentations from the Neurosurgery Spine Conference held at Stanford Hospital and Clinics on January 11, 2010. Cases include detailed histories, physical examinations, MRI findings, diagnoses, and treatment plans for patients with spinal conditions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
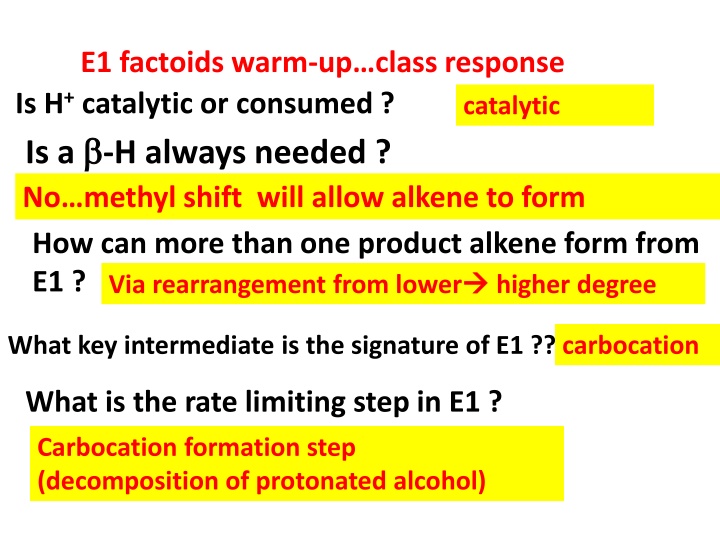
E1 factoids warm-upclass response Is H+ catalytic or consumed ? catalytic Is a -H always needed ? No methyl shift will allow alkene to form How can more than one product alkene form from E1 ? Via rearrangement from lower higher degree What key intermediate is the signature of E1 ?? carbocation What is the rate limiting step in E1 ? Carbocation formation step (decomposition of protonated alcohol)
E2 factoids warm-upclass response Is OH- catalytic or consumed ? How many atoms are involved in E2 electron transfer ? 4 consumed Is a -H always needed ? How can more than one product alkene form from E2 ? yes Non-equivalent -H on either side of halogen What starts the E2 mechanism ? Lone pair from OH- form bond to a -H, which then donates C-H bond electrons to substrate Is there a primary H effect in E2 ? Yes
`ELIMINATION ( ) ROUND Mole $ Q & A Individual response Substrate for E1 elimination ROH =alcohols Mechanism using base-driven attack E2 Mechanism has primary H effect E2 Mechanism where rate varies as: I- > Br- > Cl- > F- E2 1 How many alkenes can be formed from Cl Where s the active site(s) in: Cl
Acid catalyzed E1 is a(n) ________________ reaction dehydration In E2 elimination the OH- is: a)Catalytic and not consumed b)Substituted permanently into the product c)Consumed and converted to alcohol d)Consumed and converted to water e)A pain in my toukiss Base-driven E2 is called the _________________ reaction dehydrohalogenation
How many different alkenes can be made from: 2 OH E1 I need to take acid to work ! (E1 or E2) ? Mechanism(s) that follow Zaitsev rule for product alkene distribution Both E1 and E2 Mechanism involving a 4-atom dance with electrons E2 E1 I eat, then vomit back H+ when I eliminate
OH- H E2 elimination is initiated by attack of _______ on a_____ H+ E1 elimination is initiated by an attack of __________ on the __________________ Alcohol s OH group Which mechanism competes with E1 if nucleophiles are present ??? SN1 I eat base and crap salt when I eliminate. E2 E1 Only if no base is around will this mechanism prevail during dehydrohalogenation
Before I go to work I drink copious amounts of C2H5OH (E1 or E2?) E2 KOH No reaction ????? ethanol Cl H+ ????? reflux + OH In the products of the above, which is major ? Major
E1 factoids Is H+ catalytic or consumed ? catalytic Is a -H always needed ? No methyl shift will allow alkene to form How can more than one product alkene form from E1 ? Via rearrangement from lower higher degree What key intermediate is the signature of E1 ?? carbocation When will dehydration run E2 ??? For 1o alcohol w/conditions favoring E2 (non-polar solvent)
https://encrypted-tbn3.gstatic.com/images?q=tbn:ANd9GcQCICS8b_WCyXYAOYtDAylynrqmr0KPiImOZ2_Z1JdyoOnMYb3JFAhttps://encrypted-tbn3.gstatic.com/images?q=tbn:ANd9GcQCICS8b_WCyXYAOYtDAylynrqmr0KPiImOZ2_Z1JdyoOnMYb3JFA