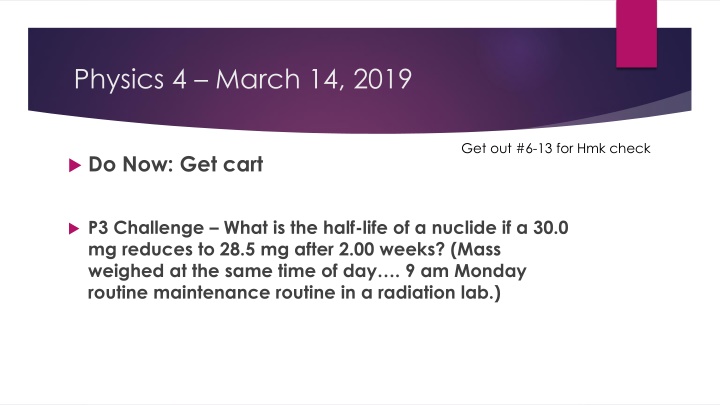
Nuclear Stability and Decay: Understanding Half-Life and Radioactive Nuclides
Explore the concepts of nuclear stability, decay reactions, and half-life in this physics lesson. Learn how to write nuclear equations, predict types of radiation, and calculate binding energy. Discover the fascinating world of nuclear physics.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Physics 4 March 14, 2019 Get out #6-13 for Hmk check Do Now: Get cart P3 Challenge What is the half-life of a nuclide if a 30.0 mg reduces to 28.5 mg after 2.00 weeks? (Mass weighed at the same time of day . 9 am Monday routine maintenance routine in a radiation lab.)
Objectives/Agenda/Assignment Agenda: Objective: 7.2 Nuclear Stability Nuclear equations for decay reactions Assignment: Binding energy and nuclear stability p294 #16, 17, 23 and Handout 7.2 Nuclear Stability Time to work with Table of Nuclides
Writing Nuclear equations for decays An alpha decay loses a helium nucleus ? daughter nucleus which can be determined by conservation of nucleon number A and charge Z. ?H He e . The other product is called a Ex: 238? 2 238? 2 4He + Z 4He + 90 A??238 = 4 + A A = 234 92 = 2 +Z Z = 90 = Thorium Th 92 92 234?? An beta decay loses an electron 1 more proton and one less neutron. Nucleon number does not change. There is also an anti-neutrino 0 Ex: 27 60?? 1 0e ..from the nucleus . Net result is there is one 0 emitted at the same time. 60?? 1 0e + Z 0e + 28 A?? + 0 60?? + 0 0 A = 60 Z -1 = 27 Z = 28 = Nickel Ni 0 27
Predicting type of radiation Based on its mass number you can predict what kind of radioactive decay a nuclide will experience. If Z > 83, alpha decay. If A > atomic mass, then it is neutron rich and will have beta decay If A < atomic mass then it is neutron poor and will have positron decay or electron capture If A is very near the atomic mass, it is likely stable. Can predict stable if both the number of neutrons (N) and the number of protons (Z) is even. 157/264 stable nuclides fit this description. Only 4/264 are odd, odd.
Mass Defect and Binding Energy The mass of a nucleus is always less than the combined masses of its nucleons. The lost mass, or mass defect, is released as energy. = Zmp + (A-Z)mn Mnucleus Mnucleus = Atomic Mass Zme Amount of energy produced from the mass defect is E = mc2. This energy is the nuclear binding energy: the energy required to break a nuclei into individual nucleons. Binding energy is usually expressed using mega eV (MeV) per nucleon. 1.66 x 10-27 kg = 1 amu = 931.5 x 106 eV = 931.5 MeV = 1.49 x 10-10 J
Calculating binding energy Iron-56 is an extremely stable nuclide. Compute the binding energy per nucleon in MeV for 56Fe. (mass of proton = 1.007276 amu; mass of neutron = 1.008665 amu; mass of electron = 0.000549 amu)
Binding Energy and nuclear stability Graph of Binding Energy per Nucleon (divide binding energy by mass number) Ni-62 is most stable The energy released in a decay comes from the energy required to bind a nucleus together.
Exit slip and homework Exit Slip Nitrogen-14 has a mass of 14.003074 u. What is the binding energy of a Nitrogen-14 nucleus in J? (Atomic number= 7) What s due? (homework for a homework check next class) p294 #16,17, 23 and Binding Energy Worksheet #1-5 What s next? (What to read to prepare for the next class) Read 7.2 p