Odd One Out - Hydrocarbons Quiz
In this activity, you will need to identify the odd one out in sets of words related to hydrocarbons and provide reasons for your choice. Additional tasks include describing the cracking process, distinguishing between alkanes and alkenes, and summarizing the importance of producing small-chain hydrocarbons.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
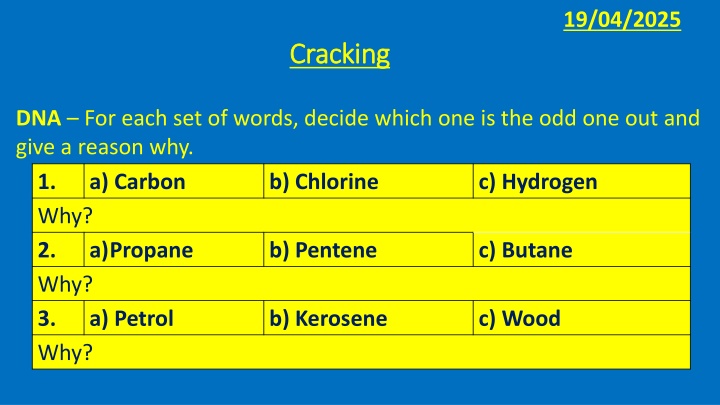
19/04/2025 Cracking Cracking DNA For each set of words, decide which one is the odd one out and give a reason why. 1. a) Carbon b) Chlorine Why? 2. a)Propane b) Pentene Why? 3. a) Petrol b) Kerosene Why? c) Hydrogen c) Butane c) Wood
Progress indicators Progress indicators Good progress: Describe how we can break down hydrocarbons to produce smaller molecules Explain how to distinguish between alkanes and alkenes Outstanding progress: Complete symbol equations to show the different products of cracking
Word consciousness Thermal decomposition breaking down using heat Alkene hydrocarbons with double carbon-carbon bond(s)
Why do we need to produce more small chain hydrocarbons? In pairs, use the graph to summarise why
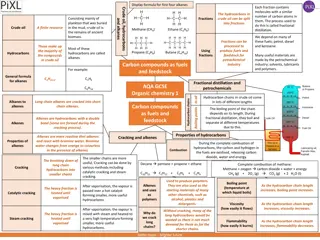
Activity 1: Describe the process of cracking Activity 1: Describe the process of cracking https://www.youtube.com/watch?v=Xsqlv4rWnEg Watch the video and answer the following: 1. Why are smaller hydrocarbons more useful? 2. What is the problem after fractional distillation? 3. What are the two products of cracking? 4. Why are alkenes useful? 5. What are the two ways cracking can occur? Extension: Could you draw two products if nonane (C9H20) was cracked?
1. Why are smaller hydrocarbons more useful? More volatile so easier to ignite therefore more useful as fuels 2. What is the problem after fractional distillation? There is a large supply of less useful long chain molecules, there is not enough short chain hydrocarbons to meet demand 3. What are the two products of cracking? A short chained alkane and an alkene 4. Why are alkenes useful? Used in the manufacture of plastics (polymers) 5. What are the two ways cracking can occur? - Thermal cracking (high temperature and pressure) - Catalytic cracking (using a catalyst)
Activity 2: Explain how to distinguish between alkanes and Activity 2: Explain how to distinguish between alkanes and alkenes alkenes Watch the demo of bromine water with an alkane and alkene then answer the questions opposite. 1. What colour is bromine water? orange 2. What happens when bromine water is added to an alkane? Nothing, remains orange 3. What happens when bromine water is added to alkene? Turns colourless 4. Bromine water was added to two substances, A and B. A did not change and B turned colourless. Which substance is an alkene? How do you know? colourless when added to it If unable to demo with bromine water, watch the following clip: B as the bromine water turned https://www.youtube.com/watch?v=PE1CDR1S5pk
Activity 3: Demonstrate the products of cracking octane Activity 3: Demonstrate the products of cracking octane In your groups, use the moly mods to build a molecule of octane (C8H16) + ? Octane Pentane Propene Octane can be broken down into two compounds, one of which is pentane (C5H12). Break your octane up to build a molecule of pentane. Using only the atoms and bonds already in octane, what is the other product?
Activity 4 Activity 4 complete the cracking equations complete the cracking equations 1. C8H18 C2H6 + ___________ 2. C10H22 C4H10 + __________ 3. C10H22 C3H6 + ___________ 4. C9H20 C4H8 + ____________ 5. C14H30 C10H20 + __________ 6. The hydrocarbon C16H34can be cracked: C16H34 ______ C2H4+ C8H18 Balance the equation for this cracking reaction. Challenge: In a cracking reaction, an alkane with 14 carbon atoms breaks down to produce two smaller hydrocarbon molecules. One is an alkane with 8 carbon atoms and the other is an alkene. Write a balanced symbol equation for this reaction.
1. C8H18 C2H6 + C6H12 2. C10H22 C4H10 + C6H12 3. C10H22 C3H6 + C7H16 4. C9H20 C4H8 + C5H12 5. C14H30 C10H20 + C4H10 6. The hydrocarbon C16H34can be cracked: C16H34 4C2H4+ C8H18 Activity 4 Activity 4 self assess self assess Challenge: In a cracking reaction, an alkane with 14 carbon atoms breaks down to produce two smaller hydrocarbon molecules. One is an alkane with 8 carbon atoms and the other is an alkene. Write a balanced symbol equation for this reaction. C14H30 C8H18+ C6H12
Exam Practice: Self assess Exam Practice: Self assess (a) and hydrogen (not mixture etc.) A compound made from carbon 1 (b) C5H12 1 (c) (i) by heat 1 (ii) Speeds up reaction 1 (iii) C8H16 1 Break down 1
Plenary Plenary- - quiz master quiz master Come up with 3 quiz questions based on today s lesson. Swap your book with the person next to you and see if they can correctly answer the questions.