One-Year Outcomes from ACURATE neo2 PMCF Registry Study
Study on ACURATE neo2, a 2nd-generation aortic valve system, focusing on 1-year outcomes in routine clinical practice. Key aspects include safety endpoints, core laboratory evaluations, and patient follow-up data. The study highlights improvements over the prior-generation ACURATE neo, with a specific emphasis on reducing paravalvular leak (PVL) and enhancing patient outcomes.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
One-year outcomes from the ACURATE neo2 PMCF Post-market Registry Won-Keun Kim, MD Kerckhoff-Klinik GmbH, Bad Nauheim, Germany On behalf of the ACURATE neo2 Post-Market Clinical Follow-up Study Investigators CAUTION: In Europe, ACURATE neo and neo2 Aortic Valve Systems are CE-marked. In the USA, ACURATE neo2 is an investigational device and restricted under federal law to investigational use only. Not available for sale. SH-1743303 AA
Potential conflicts of interest Speaker's name : Won-Keun Kim I have the following potential conflicts of interest to declare: Receipt of grants / research support: Boston Scientific Receipt of honoraria or consultation fees: Abbott, Boston Scientific, Edwards Lifesciences, Meril, Shockwave Medical PCRLondonValves.com SH-1743303 AA
Why this study? ACURATE neo2is a 2nd-generation supra-annular valve that features improvements to further reduce PVL compared to the prior-generation ACURATE neo. CE marked 2020 Self-expanding nitinol frame Top-down deployment The PMCF post-market registry studied neo2 in routine clinical practice. Porcine pericardial leaflets in a supra-annular position Enhanced sealing skirt Outcomes at 30 days have been previously presented. Here, we report 1-year outcomes. PCRLondonValves.com SH-1743303 AA
What did we study? Prospective multicentre single-arm post-market surveillance study in a routine clinical practice setting Independent CEC adjudication of safety events Primary safety endpoint: 30-day all-cause mortality Additional endpoints evaluated through 1 year Death, stroke, bleeding, major vascular complications, hospitalization for valve-related symptoms Independent core laboratories Echocardiography: MedStar Health Research Institute Hemodynamics and PVL at discharge, 30 days, and 1 year 4D-CT: The University of British Columbia, Department of Radiology Hypo-attenuated leaflet thickening (HALT) at 30 days [primary imaging endpoint] and 1 year PCRLondonValves.com SH-1743303 AA
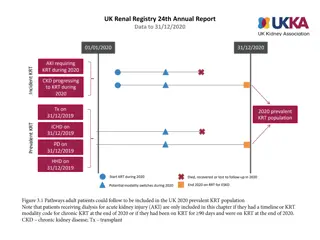
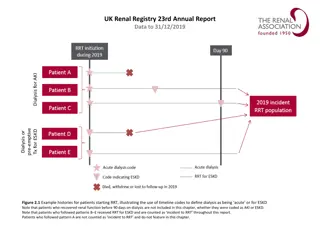
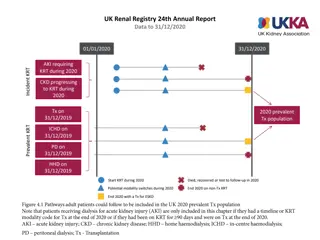
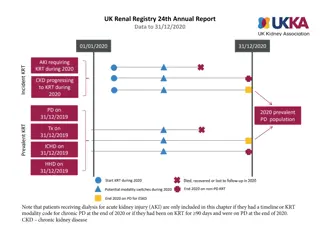
How was the study executed? Intention-To-Treat N=250 Successfully Implanted N=246 Mean Age 80.8 6.2 yrs Device not in correct position n=4* 36.4% Male 63.6% Female 30-day Imaging Data Analyzed 30-day Clinical Follow-up Visit Completed or Death 94% (236/250) TTE: 91% (224/246) CT: 83% (204/246) Mean STS Score 2.9% 2.0% Premature discontinuation Withdrawal from study, n=20 Missed 1y follow-up, n=7 Operative Risk (per Heart Team) Low 1-year Imaging Data Analyzed High 37.2% 1-year 31.2% Clinical Follow-up Visit Completed or Death 89% (223/250) TTE: 76% (186/246) CT: 62% (153/246) Intermediate 31.6% *In 4 patients, ACURATE neo2 embolized and patients were implanted with a non-study valve; these patients were followed for safety only through 30 days Patient survival confirmed, but no additional follow-up data collected. SH-1743303 AA
What are the essential results? Key Safety Events All-cause mortality Cardiovascular mortality All stroke Major bleeding Hospitalization for valve-related symptoms or worsening CHF Prosthetic aortic valve thrombosis Prosthetic aortic valve endocarditis Valve-related repeat procedure (BAV) Discharge 0.4% (1) 30 Days 0.8% (2)* 1 Safety Endpt 1 Year 5.1% (12) 0.4% (1) 0.8% (2) 3.4% (8) 0.4% (1) 0.8% (2) 3.0% (7) 2.4% (6) 2.4% (6) 2.8% (7) 0.0% (0) 0.0% (0) 1.7% (4) 0.9% (2) 1.7% (4) 0.4% (1)^ 0.4% (1) 0.0% (0) 0.4% (1) 0.4% (1)* 0.8% (2) 0.4% (1) Newly implanted permanent pacemaker 5.2% (13) 6.1% (15) 7.8% (19) *Deaths: 1 patient experienced life-threatening bleeding (admitted to secondary hospital, source of bleeding not recorded) and hemodynamic instability leading to circulatory arrest and death; 1 patient died post index procedure following valve embolization and unsuccessful TAV-in-TAV with non-study valve leading to coronary obstruction and procedural myocardial infarction. *Surgical removal of embolized ACURATE neo2 valve ^Thrombus noted pre-discharge in non-study valve implanted subsequent to ACURATE neo2 embolization Diagnosis of HALT at 30 days and 1 year, with oral anticoagulant therapy initiated shortly before 1-year visit PCRLondonValves.com SH-1743303 AA
What are the essential results? Echocardiography Core Laboratory Aortic Valve Haemodynamics, ITT Population Paravalvular Leak, Paired Analysis (n=116) 100 2.0 1.7 0.4 (n=134) 1.6 0.4 (n=139) 1.6 0.4 (n=127) 0.9 1.9 15.3 16.2 22.6 80 1.6 Mean AV Gradient (mmHg). Severe Mean AV Area (cm2) 47.6 14.5 (n=240) 60 1.2 Moderate Mild 40 0.8 83.8 81.9 77.4 None/Trace 0.7 0.2 (n=237) 9.7 5.4 (n=193) 8.6 3.9 (n=180) 7.6 3.2 (n=169) 20 0.4 0 0.0 Baseline Discharge 30 Days 1 Year Discharge/7d 30 Days 1 Year PCRLondonValves.com SH-1743303 AA
What are the essential results? CT Core Laboratory HALT, Paired Analysis (n=150) HALT @ 30d or 1y N=72 0.0% (0) No HALT @ 30d and 1y N=93 0.0% (0) 1-Year Event Rate P-value 30 Days 1 Imaging Endpt 76.0% 14.6% 9.4% All-cause mortality 0.25 HALT severity 50% HALT severity >50% CV mortality 0.0% (0) 0.0% (0) 0.25 No HALT All stroke 2.2% (2) 2.2% (2) 0.74 1 Disabling stroke 0.0% (0) 0.0% (0) -- 68.0% 20.6% 11.4% Year Valve thrombosis 0.0% (0) 0.0% (0) 0.25 Echocardiography n=27* n=91 Change in HALT severity from 30 days 1 year AV gradient (mm Hg) 7.1 4.3 7.8 2.7 0.36 No change Improved 12% AV area (cm2) 1.6 0.4 1.7 0.4 0.53 67% *Only subjects with HALT recorded at 30 days and 1 year are included in the analysis. 21% (n=135, excludes pts on OAC) Worsened PCRLondonValves.com SH-1743303 AA
The essentials to remember This report confirms favorable performance and safety through 1 year in patients with severe aortic stenosis treated with ACURATE neo2 Patients treated with ACURATE neo2 exhibited early improvement in valve haemodynamics and low rates of PVL through 1 year At 1 year, 99% of patients had mild or no/trace PVL (<1% had moderate PVL) No patients exhibited >moderate PVL at any time 1-year rates of all-cause mortality (5.1%) and stroke (3.0%) are comparable to other trials of TAVR patients at intermediate or high surgical risk The presence of HALT: Did not impact patient safety at 1 year Did not significantly affect mean AV gradient or mean AV area PCRLondonValves.com SH-1743303 AA
PCRonline.com CAUTION: In Europe, ACURATE neo and neo2 Aortic Valve Systems are CE-marked. In the USA, ACURATE neo2 is an investigational device and restricted under federal law to investigational use only. Not available for sale.