Organic Compounds, Alkanes, and Fractional Distillation
Explore the world of organic chemistry, focusing on the origin of crude oil, hydrocarbon compounds, their families, and the process of fractional distillation. Learn about alkane structures, properties, and uses in this comprehensive guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Organic Compounds the origin of crude oil and its use as a source of hydrocarbon raw materials the grouping of hydrocarbon compounds into families (alkanes, alkenes, alkynes, alcohols, carboxylic acids and non-branched esters) based upon similarities in their physical and chemical properties including general formulas, their representations (structural formulas, condensed formulas, Lewis structures), naming according to IUPAC systematic nomenclature (limited to non-cyclic compounds up to C10, and structural isomers up to C7) and uses based upon properties determination of empirical and molecular formulas of organic compounds from percentage composition by mass and molar mass.
Organic Chemistry Carbon is an unusual atom in that it is able to form four very strong covalent bonds with other carbon atoms. When we then include it s ability to also bond with other elements we open up the possibility of the highly diverse and complex molecules (like DNA) that have led to the possibility of life. Because of this, the chemistry of carbon containing compounds is often called organic chemistry.
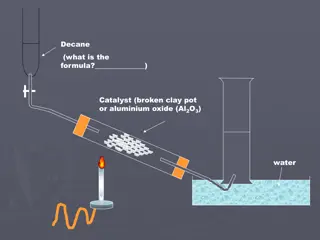
Crude oil and alkanes Crude oil is a mixture composed mainly of straight and branched chain alkanes. It also includes lesser amounts of cycloalkanes and arenes, both of which are hydrocarbons containing a ring of carbon atoms, as well as impurities such as sulfur compounds. The exact composition of crude oil depends on the conditions under which it formed, so crude oil extracted at different locations has different compositions.
What are alkanes? The alkanes are a homologous series of hydrocarbons with the general formula CnH2n+2 and names ending ane. Alkanes contain only single carbon carbon bonds and so are saturated. No. of Molecular formula Name carbon atoms CH4 1 methane C2H6 ethane 2 C3H8 3 propane C4H10 butane 4 C5H12 C6H14 pentane hexane 5 6
What are alkanes? H H C methane, CH4 H H H H H propane, C3H8 C C H C H H H H H H H H H C C C H C C H H H H H H pentane, C5H12
Representing organic molecules The molecular formula of a compound shows the number of each type of atom present in one molecule of the compound. Molecular formula Empirical formula C2H6 CH3 C6H12O6 CH2O The empirical formula of a compound shows the simplest ratio of the atoms present. C2H4O2 CH2O Neither the molecular nor empirical formula gives information about the structure of a molecule.
Structural formula - full The full structural formula of a compound shows the arrangement of atoms in a molecule, as well as all the bonds. Single bonds are represented by a single line, double bonds with two lines and triple bonds by three lines The full structural formula can show the different structures of compounds with the same molecular formulae. ethanol (C2H6O) methoxymethane (C2H6O)
Structural formula - condensed The condensed structural formula shows all the atoms and their relative positions but bonds are omitted. Double or triple bonds may be shown. CH3CHClCH3 H2C=CH2 CH3C N 2-chloropropane ethene ethanenitrile
Structural formula - skeletal The skeletal formula of a compound shows the bonds between carbon atoms, but not the atoms themselves. Hydrogen atoms are also omitted, but other atoms are shown.
What is isomerism? Isomers are molecules with the same molecular formula (i.e. the same number and type of atoms) but in which the atoms are arranged in a different way. For now we will learn about structural isomerism Structural isomers have the same molecular formula but different structural formulae. Three types of structural isomerism are chain isomerism, positional isomerism and functional group isomerism.
Chain isomerism in alkanes In chain isomers, the carbon chain is arranged differently. For example, hexane has several chain isomers, all with the molecular formula C6H14: hexane 2,3-dimethylbutane 3-methylpentane
Naming alkanes 1. Number and name the longest chain of carbon atoms 2. Look for the branches. di-, tri etc used for more than one branch of same kind. Write branches in alphabetical order 3. Find the number of the carbon atom to which the branch is attached. (Number from whichever end gives the lowest numbers) 4. Complete by adding the number and name of the branch to the carbon chain. Put comma's between numbers eg 2,2 or 2,3. Hyphens separate numbers from letters eg 2,2-dimethyl. No gaps between names eg methylpropane
Complete combustion In excess oxygen, short chain alkanes can undergo complete combustion: alkane + oxygen carbon dioxide + water For example: propane + oxygen carbon dioxide + water C3H8(g) + 5O2(g) 3CO2(g) + 4H2O(g) The combustion of alkanes is a highly exothermic process. This makes them good fuels because they release a relatively large amount of energy per gram of fuel.
Incomplete combustion If oxygen is limited then incomplete combustion will occur: alkane + oxygen carbon monoxide + water alkane + oxygen carbon + water For example: propane + oxygen carbon monoxide + water C3H8(g) + 3 O2(g) 3CO(g) + 4H2O(g) propane + oxygen carbon + water C3H8(g) + 2O2(g) 3C(s) + 4H2O(g)