Overview of Amines: Structures, Reactions, and Functional Groups
Amines are basic nitrogen compounds classified as ammonia, primary, secondary, tertiary, quaternary amines based on their structure. These reactive compounds undergo various reactions like alkylation, acylation, salt formation, and oxidation. Additionally, other nitrogen-containing functional groups such as oximes, nitrates, nitrites, and hydroxamic acids are discussed, highlighting their toxic nature and chemical properties. Explore a comprehensive guide to amines and related compounds in organic chemistry.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
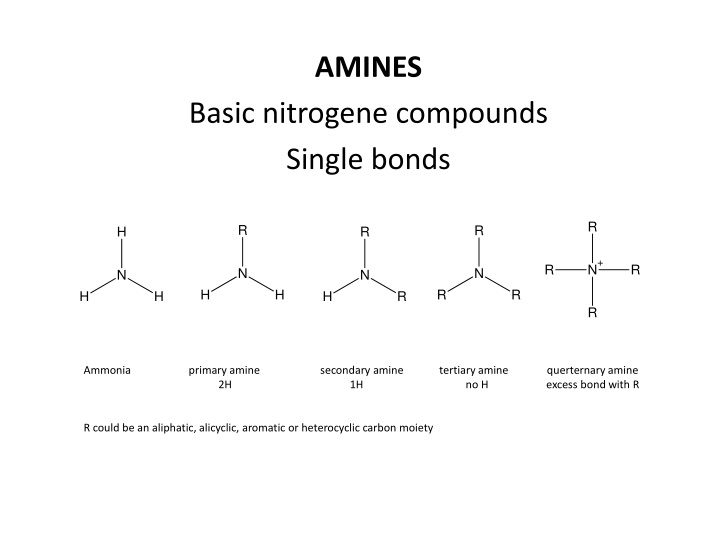
AMINES Basic nitrogene compounds Single bonds R R R H R N+ R R N N N N H H R R H H H R R Ammonia primary amine secondary amine tertiary amine querternary amine 2H 1H no H excess bond with R R could be an aliphatic, alicyclic, aromatic or heterocyclic carbon moiety
-NH2 (amino) group a polar and basic group -Amines are highly reactive -Nomenclature: Simply indicate the hydrocarbone moiety first and then amine ending: CH3-NH2 methylamine C6H5-NH2 aniline C2H5 N-methylethylamine (small group as a substituent) N H CH3 C2H5 N,N-dimethylethylamine N H5C2 CH3 CH3 N N-ethyl-N-methylaniline C2H5
Amines are synthetic intermediates but they are rather toxic chemicals. Their important reactions: Alkylation: R RX RX R N R NH R NH2 R R RX R N+ R R R
Acylation: (with acyl chlorides to give amides): RCOX R COR R NH2 (R) NH (R) Salt formation (with acids): HCl +.Cl- R NH2 (R) R NH3 (R) Imine formation: (with aldehydes or ketones): R R R + O R NH2 N - H2O R (H) R
Oxidation: (O) R OH R NH2 (R) NH (R) primary and secondary amines give hydroxylamines R R (O) N N R R R R O 0 tertiary amines give N-oxides
Other functional groups consisting of nitrogene Oximes R-CH=N-OH CH3-CH=N-OH acetaldehyde oxime C6H5-CH=N-OH benzaldehyde oxime (CH3)2C=N-OH acetone oxime Nitrates (nitric acid esters) R-O-NO2 CH3-O-NO2 methyl nitrate C6H5-O-NO2 phenyl nitrate
Nitrites (nitrous acid esters) R-O-NO CH3-O-NO methyl nitrite C6H5-O-NO phenyl nitrite Hydroxamic acids R-CO-NH-OH Very toxic compounds These can be considered as N-hydroxiyamides or acyl/aroyl hydroxylamines C6H5-CO-NH-OH hydroxybenzamide C6H5-CO-NH-OH hydroxybenzamide benzohydroxamic acid or N- benzohydroxamic acid or N-
Oxidation of amides are difficult compared to amines but in case it is possible, hydroxamic acids can be obtained. Generally aliphatic amides are much more easily oxidized: O O (O) R NHOH R NH2 HYDRAZINE DERIVATIVES Hydrazine is a very basic, small compound H2N-NH2 Hydrazine can be alkylated, acylated as in the case of amines to orm some derivatives: NH2-NH-CH3 N-methylhydrazine
N-acylhydrazines are known as hydrazides and these are important antimicrobial agents in medicinal chemistry: NH-NH-CO-CH3 acetic acid hydrazide or acetic hydrazide or aceto hydrazide O NHNH2 ISONIAZID (isonicotinic acid hydrazide) N O NHNH2 p-aminobenzoic hydrazide NH2
Ring hydrazide is also important: NH N H O IMINES -C=N- is imino group NH NR (Ar) N-nonsubstituted imine N-substituted imine Stability order: N-aromatic substituted > N-aliphatic substituted > N-nonsubstituted imines N-aromatic substituted imines are called Schiff Bases
CH3-CH=N-H 1-etanimine (CH3)2C=N-H 2-propanimine N-substituted imines are named as N-alkylidene or N-arylidene substituted amines: C6H5-N=CH-C6H5 CH3-N=CH-CH3 N-benzylideneaniline N-ethylidenemethylamine Imines can be easily hydrolyzed back to amine plus the corresponding carbonyl compound (aldehyde or ketone): R-N=CH-R + H2O -> R-NH2 + O=CH-R
HYDRAZONES These are products of hydrazines with aldehydes and ketones: R-CHO + H2N-NH2 -> R-CH=NH-NH2 (hydrazones) (R) (R) These compounds have generally antimicrobial property. Nomenclature: CH3-CH=N-NH2 acetalhydrazone (acetaldehyde hydrazone) C6H5-CH=N-NH2 benzalhydrazone (benzaldehyde hydrazone)
NITRILE (CYANIDE) R C N Toxic moiety -CN cyano moiety Their names come from the name of the corresponding acids: CH3-CN acetonitrile (from acetic acid) C6H5CN benzonitrile (from benzoic acid) Alternatively, nitriles can be named as cyanides (names are methyl cyanide or phenyl cyanide for the compounds above) Nitriles can be hydrolysed to carboxylic acids via amides: RCN + H2O -> R-CONH2 R-CONH2 + H2O -> R-COOH
AZO COMPOUNDS R-N=N-R symmetrical R-N=N-R1 asymmetrical CH3-N=N-CH3 azomethane C3H7-N=N-C3H7 azopropane CH3-CH2-N=N-CH3 methylazoethane C6H5-N=N-CH3 methylazobenzene (in assymetric ones, the smaller carbon is considered as a substituent)
AZOXY COMPOUNDS O N R R N azoxybenzene O N C6H5 H5C6 N N-NITROSAMINES N-nitrosoaniline C6H5-NH-NO NITRO COMPOUNDS R-NO2 CH3-NO2 nitromethane C6H5-NO2 nitrobenzene
NITROSO COMPOUNDS R-NO CH3-NO nitrosomethane C6H5-NO nitrosobenzene
NITRIC ACID ESTERS (NITRATES) R-O-NO2 CH3-O-NO2 C6H5-O-NO2 phenyl nitrate methyl nitrate NITROUS ACID ESTERS (NITRITES) R-O-NO CH3-O-NO C6H5-O-NO methyl nitrite phenyl nitrite
NITRONES (This group can be considered as N-oxide of imines) R(H) + R N - R(H) O alpha carbon CH3 + H5C2 N N-ethyl-alpha-methyl-alpha-propylnitrone - C3H7 O O N,alpha-diphenylnitrone N
UREA and DERIVATIVES HO-CO-OH carbonic acid NH2-CO-OH carbamic acid NH2-CO-NH2 carbamide (urea) Urea is a monoamide of carbamic acid and a diamide of carbonic acid Different nitrogenes can be named either N, N or N1, N3 and these can be substituted with alkyl, aryl or other functonal groups. NH2-CO-NH-CH3 (N-methyl urea) Cl-NH-CO-NH-C2H5 (N-chloro-N -ethylurea)
UREIDES (N-acylurea) NH2-CO-NH-CO-R NH2-CO-NH-CO-CH3 (N-acetylurea) Cyclic ureide is also important in pharmaceutical chemistry: O H N NH O O Malonylurea (BARBITURIC ACID)
CARBAMATES (URETANE) NH2-CO-OR Carbamic acid esters are called as carbamates or uretanes NH2-CO-OC2H5 is ethyl uretane or ethyl carbamate
THIOUREA H2 N NH2 S SEMICARBAZIDE (N-aminourea) NH2-CO-NH-NH2 1 2 3 4 SEMICARBAZONE (N-aminourea) NH2-CO-NH-NH=CH(R)-R NH2-CO-NH-NH=CH-CH3 (acetaldehydesemicarbazone)
THIOSEMICARBAZIDES (N-aminourea) NH2-CS-NH-NH2 1 2 3 4 THIOSEMICARBAZONES (N-aminourea) NH2-CS-NH-NH=CH(R)-R NH2-CO-NH-NH=CH-C6H5 (benzaldehydethiosemicarbazone)
IMPORTANT SULPHUR CONTAINING FUNCTIONAL GROUPS THIOALCOHOLS R-SH CH3-SH methanethiol THIOPHENOLS C6H5-SH THIOETHERS (SULFIDES) R-S-R CH3-S-CH3 (dimethylsulfide) C2H5-S-C6H5 (ethylphenylsulfide)
SULFOXIDES R-SO-R CH3-SO-CH3 dimethylsulfoxide SULFONES R-SO2-R CH3-SO2-CH3 dimethylsulfone (O) (O) R R O R R S S S O R O R
SULFONIC ACIDS R-SO3H CH3-SO3H methanesulfonic acid C6H5-SO3H benzenesulfonic acid O O (O) (O) S (O) S R SH R SOH R OH R OH O
SULFONAMIDES R-SO2-NH2 Arylsulfonamides are important antimicrobial agents. NH2 SO2NH2 p-aminobenzenesulfonamide
SULFONYL CHLORIDE R-SO2-Cl Sulfonyl chlorides are important SIM for producing sulfonamides C2H5-SO2-Cl ethanesulfonyl chloride C6H5-SO2-Cl benzenesulfonyl chloride SULFONYLUREA R-SO2-NH-CO-NH2 Sulfonylureas are important antihyperglycemic compounds: CH3 SO2NHCONHC4H9 1-butyl-3-p-(tolylsulfonyl)urea
THIONES Thiones are ring ketones with S (with the replacement of O in ketones): S Thiocyclohexanone (cyclohexanethione)
POLIFUNCTIONAL GROUPS Polyfunctional groups may contain more than one functional group in a molecule. One of them is selected as a main functional group and the rest is considered as substitents. The order of their priority is as follows: carboxylic acid > carboxylicacid derivatives > aldehydes and ketones > alcohol > amine > double bond For example: H2N-CH2-CH2-CH2-CH2-OH 4-amino-1-butanol (not 1-amino-4-butanol)
a. Unsaturated acids CH3-CH=CH-COOH 2-butenoic acid (crotonic acid) CH2=CH-COOH 2-propenoic acid (acrylic acid) b. Unsaturated aldehydes CH2=CH-COOH acrylic acid CH2=CH-CHO acrolein CH3-CH=CH-COOH crotonic acid CH3-CH=CH-COOH crotonaldehyde
c. Hydroxy acids CH3-CH(OH)-COOH lactic acid d. Amino acids NH2-CH2-COOH glycine CH3-CH(NH2)-COOH alpha-alanine H2N-CH2-CH2-COOH beta-alanine
e. Hydroxyaldehydes CHO (CHOH)4 CH2OH glucose f. Polyoles (dioles, trioles) CH2OH CH2OH Ethylenglycol (GLYCOL) or 1,2-ethanediol CH2OH CHOH CH2OH Glycerol (GLYCERIN) or 1,2,3-propanetriol
g. Diacids COOH COOH oxalic acid COOH CH2 COOH malonic acid COOH CH2 CH2 COOH succinic acid
IMPORTANT REACTIONS IN MEDICINAL CHEMISTRY 1. Alkylation (amines) 2. Acylation (amines) 3. Halogenation (aromatic substitution) 4. Esterification (carboxylic acid, alcohol) 5. Sulphonation (aromatic substitution) 6. Nitration (aromatic substitution) 7. Schiff base formation (imines, amines, aldehydes, ketones) 8. Oxidation (Aldehyde, ketone, alcohol, amine) 9. Reduction (aldehyde, ketone, nitro, azo,double bond) 10. Hydrolysis (ester, amide, imine) 11. Decarboxylation (carboxylic acid) 12. Dealkylation (pr and sec amine, ether, thioether)
AROMATIC SUBSTITION OF BENZENE RING O N+ O O- OH S O nitration, , HNO3 sulfation, H2SO4 X , halogenation R-Cl, alkylation, R-CO-Cl acylation COR R
1st group substituents These are ortho and para directing groups ie OH, NH2, X and CH3 Reactions are easier compared to 2nd group substituents: CH3 CH3 Cl2 Cl Cl 2,4,6-trichlorotoluene Cl 2st group substituents These are metadirecting groups ie COOH, and NO2 groups Reactions are more difficult compared to 1st group substituents: NO2 NO2 HNO3 Cl Cl 3,5-dichloronitrobenzene