Overview of HCC Experts Round Table - Key Data & Guidelines
The HCC Experts Round Table in Asia-Pacific discussed key data on hepatocellular carcinoma (HCC) in July 2020. The round table covered treatment choices, management of advanced HCC patients, and the impact of IMbrave150 data in clinical practice. Additionally, it showcased Asian HCC guidelines from various countries, highlighting epidemiology, prevention, surveillance, diagnosis, treatment, and follow-up protocols. The summary presented insights from experts representing different specialties and regions, offering valuable perspectives on addressing the challenges of advanced HCC management and care.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
HCC EXPERTS ROUND TABLE (ASIA-PACIFIC) Prof. Pierce Chow MBBS MMed, FAMS, FRCSE, PhD National Cancer Centre Singapore and Singapore General Hospital Singapore OVERVIEW OF KEY DATA July 2020 HCC, hepatocellular carcinoma 2
DISCLAIMER Please note: Views expressed within this presentation are the personal opinions of the author. They do not necessarily represent the views of the author s academic institution, organisation, or other group or individual. This content is supported by an Independent Educational Grant from Roche. Disclosures: Prof. Pierce Chow has received honoraria from the following: Sirtex Medical, Ipsen, Bristol-Myers Squibb, Oncosil, Bayer, New B Innovation, MSD, BTG Plc, Guerbet, Perspectum, IQVIA, Genentech, Roche, AstraZeneca, AUM Biosciences, L.E.K. Consulting Pte Ltd. 3
EXECUTIVE SUMMARY The HCC Experts Round Table took place as two virtual meetings on 6 and 7 July 2020 With 7 Experts from the Asia-Pacific region: 1 Patient advocate 1 Payer/health economics expert 5 Physicians (representing hepatology, medical oncology, and surgery) 26 questions discussed: 11 questions related to treatment of choice in advanced 1L HCC in Asia (sorafenib and lenvatinib) 6 questions related to the management of advanced HCC patients in Asia (e.g. clinical setting, costs, multidisciplinary tumour board) 9 questions related to IMbrave150 data and potential impact in clinical practice in Asia Next step: Building a manuscript to reflect consensus outcomes 1L, first-line; HCC, hepatocellular carcinoma 4
INTRODUCTION AND TREATMENT OVERVIEW OF ADVANCED HCC HCC, hepatocellular carcinoma 5
HEPATOCELLULAR CARCINOMA: ASIAN HCC GUIDELINES Content Guidelines (country) Year Epidemiology Prevention Surveillance Staging & diagnosis Treatment Follow up AOS consensus1 (Asian) 2009 Chinese guidelines1 (China) 2011 JSH guideline1 (Japan) 2014 Korean guidelines1 2015 Hong Kong consensus1 2015 NCCSCG1 (Singapore) 2016 APASL consensus guidelines1 (Asian) 2017 Taiwanese consensus2 (Taiwan) 2017 AOS; Asian Oncology Summit; APASL, Asian Pacific Association for the Study of the Liver; HCC, hepatocellular carcinoma; JSH, Japan Society of Hepatology; NCCSCG, National Cancer Centre Singapore Consensus Guidelines for HCC 1. Tang H, et al. Transl Cancer Res. 2017;6:1214-225; 2. Lu S-N, et al. J Formos Med Assoc. 2018;117:381-403 6
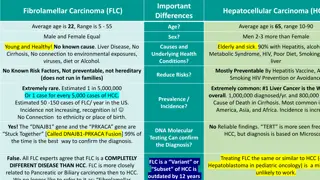
HEPATOCELLULAR CARCINOMA: OVERVIEW The fourth most common cause of cancer-related death worldwide1 HCC accounts for >80% of primary liver cancers worldwide1 Chronic HBV and HCV infection are the most important causes of HCC and account for 80% of HCC cases globally1 It is estimated that 72% of cases occur in Asia (more than 50% in China)2 Staging of HCC is important to determine outcome and planning of optimal therapy. While there are a number staging systems used, the BCLC is currently commonly used to compare clinical outcomes:3 Survival rate with current therapy >5 years >2.5 years >1 year 3 months BCLC staging Standard of care treatment Stage 0-A Stage B Stage C Stage D Ablation, resection, transplantation Chemoembolisation (TACE) Systemic therapy Best supportive care Early and intermediate HCC Advanced HCC BCLC, Barcelona Clinic Liver Cancer; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; TACE, transarterial chemoembolisation 1. Yang JD, et al. Nat Rev Gastroenterol Hepatol. 2019;16:589-604 2. Singal AG, et al. J Hepatol. 2020;72:250-61 3. Bruix J, et al. Nat Rev Gastroenterol Hepatol. 2019;16:617-30 7
SYSTEMIC TREATMENT SEQUENCING FOR BCLC STAGE C ADVANCED HCC Targeted first-line therapies Combination: atezolizumab (PD-L1 inhibitor) + bevacizumab*(VEGF inhibitor) (US only) Oral multikinase inhibitors: sorafenib and lenvatinib Targeted second-line therapies Multikinase inhibitor: regorafenib Multikinase inhibitor: cabozantinib Anti-VEGFR (AFP 400 ng/mL) antibody: ramucirumab PD-1 inhibitors: nivolumab, pembrolizumab (US only) Immune therapy Combination: nivolumab + ipilimumab (US only) First line Second line regorafenib Third line atezolizumab + bevacizumab1 cabozantinib ramucirumab nivolumab pembrolizumab nivolumab + ipilimumab1 AFP 400 ng/mL cabozantinib sorafenib lenvatinib *The combination of atezolizumab + bevacizumab was approved by the US FDA on May 29. 2020 AFP, alpha-fetoprotein; BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; PD-1, programmed death protein 1; PD-L1, programmed death-ligand 1; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor Source: Bruix J, et al. Nat Rev Gastroenterol Hepatol. 2019;16:617-30 8
SORAFENIB / LENVATINIB EFFICACY AND SAFETY DATA IN 1L FOR ADVANCED HCC PATIENTS 1L, first-line; HCC, hepatocellular carcinoma 9
SORAFENIB EFFICACY DATA Based on results from: SHARP (NCT00105443): phase 3, international, multicentre, randomised, double blind, placebo-controlled study in 602 patients with hepatocellular carcinoma Primary endpoint: OS Secondary endpoint: TTP Population enrolled: BCLC stage (stage B: 18.1% vs. 16.8%; stage C: 81.6% vs. 83.2%; stage D: <1% vs. 0%) in sorafenib and placebo respectively Efficacy parameter Sorafenib (n=299) Placebo (n=303) P-value HR (95% CI) Median OS, months (95% CI) 10.7 7.9 0.69 0.00058 (9.4-13.3) (6.8-9.1) (0.55-0.87) Median TTP, months (95% CI) 5.5 2.8 0.58 0.000007 (4.1-6.9) (2.7-3.9) (0.45-0.74) Formulation: Film-coated tablets 200 mg Recommended daily dose: 400 mg (2 200 mg tablets) twice daily BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; HR, hazard ratio; OS, overall survival; TTP, time to progression Sources: sorafenib summary of product characteristics dated November 2019; sorafenib US prescribing information dated June 2020 10
LENVATINIB EFFICACY DATA Lenvatinib N=478 Sorafenib N=476 Efficacy parameters Overall survival Number of deaths (%) Median OS in months (95% CI) Hazard ratio (95% CI) Progression-free survival (mRECIST) Number of events (%) Median PFS in months (95% CI) Hazard ratio (95% CI) and P-value Objective response rate (mRECIST) Objective response rate Complete responses, n (%) Partial responses, n (%) 95% CI P-value Progression-free survival (RECIST 1.1) Number of events (%) Median PFS in months (95% CI) Hazard ratio (95% CI) Objective response rate (RECIST 1.1) Objective response rate Complete responses, n (%) Partial responses, n (%) 95% CI Based on results from: 351 (73) 13.6 (12.1-14.9) 350 (74) 12.3 (10.4-13.9) REFLECT (NCT01761266): phase 3, international, multicentre, open-label, randomised study in 954 patients with hepatocellular carcinoma 0.92 (0.79-1.06) 311 (65) 7.3 (5.6-7.5) 0.64 (0.55-0.75); <0.001 323 (68) 3.6 (3.6-3.7) Non inferiority assessment of lenvatinib vs. sorafenib for OS 41% 10 (2.1) 184 (38.5) (36-45) 12% 4 (0.8) 55 (11.6) (10-16) Primary endpoint: OS Secondary endpoints: PFS, ORR (mRECIST and RECIST v1.1) <0.001 Population enrolled: BCLC stage B: 20%; stage C: 80% 307 (64) 7.3 (5.6-7.5) 320 (67) 3.6 (3.6-3.9) Formulation: hard capsules 4 mg or 10 mg 0.65 (0.56-0.77) Recommended dose daily: 12 mg (body weight 60 kg) or 8 mg (<60 kg) 19% 2 (0.4) 88 (18.4) (15-22) 7% 1 (0.2) 30 (6.3) (4-9) BCLC, Barcelona Clinic Liver Cancer; CI, confidence interval; mRECIST, modified RECIST; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumours Sources: lenvatinibsummary of product characteristics dated June 2020; lenvatinibUS prescribing information dated February 2020 11
SORAFENIB AND LENVATINIB SAFETY DATA IN HCC PATIENTS Most common adverse reactions ( 20%) sorafenib-treated patients in SHARP trial Diarrhoea fatigue hand-foot skin reaction weight loss anorexia nausea abdominal pain lenvatinib-treated patients in REFLECT trial Hypertension fatigue diarrhoea decreased appetite arthralgia/myalgia decreased weight abdominal pain palmar-plantar erythrodysesthesia syndrome proteinuria dysphonia haemorrhagic events hypothyroidism nausea Further and more detailed information about safety profile of both products and their management can be found in the European SmPC and USPI HCC, hepatocellular carcinoma; SmPC, summary of product characteristics; USPI, US prescribing information Sources: sorafenib SmPC November 2019; sorafenib USPI June 2020; lenvatinib SmPC June 2020; lenvatinib USPI February 2020 12
IMbrave150: A STUDY OF ATEZOLIZUMAB IN COMBINATION WITH BEVACIZUMAB COMPARED WITH SORAFENIB IN PATIENTS WITH UNTREATED LOCALLY ADVANCED OR METASTATIC HCC ClinicalTrials.gov Identifier: NCT03434379 HCC, hepatocellular carcinoma 13
IMbrave150 CLINICAL TRIAL DESIGN IMbrave150 (NCT03434379): randomized phase 3 trial assessing combination therapy with the PD-L1 inhibitor atezolizumab and the VEGF inhibitor bevacizumab versus standard-of-care sorafenib in first line for advanced HCC Stratification criteria Key eligibility criteria Locally advanced metastatic or unresectable HCC ECOG PS 0-1 No prior systemic therapy No bleeding or high risk of bleeding atezolizumab 1200 mg IV q3w + bevacizumab 15 mg/kg IV q3w Region: Asia (excluding Japan) or rest of world Until loss of clinical benefit or unacceptable toxicity Survival follow-up R 2:1 ECOG PS 0 or 1 (Open label) Presence or absence of macrovascular invasion or extrahepatic spread sorafenib 400 mg BID (n=501) Baseline AFP <400 or 400 ng/mL Co-primary endpoints OS IRF-assessed PFS per RECIST 1.1 Secondary endpoints include IRF-assessed ORR per RECIST 1.1 and HCC mRECIST PROs AFP, alpha-fetoprotein; BID, twice a day; ECOG PS; Eastern Cooperative Oncology Group performance status; HCC; hepatocellular carcinoma; IRF, independent review facility; IV, intravenous; mRECIST, modified RECIST; ORR, objective response rate; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PRO, patient-reported outcome; q3w, every 3 weeks; R, randomization; RECIST, Response Evaluation Criteria In Solid Tumours; VEGF, vascular endothelial growth factor Finn RS, et al. N Engl J Med. 2020;382:1894-905 14
IMbrave150 CLINICAL TRIAL EFFICACY RESULTS: PRIMARY ENDPOINTS atezolizumab + bevacizumab (n=336) sorafenib (n=165) 13.2 Median OS (95% CI), months NE (10.4 NE) 0.58 OS, HR (95% CI) (0.42 0.79) P-value <0.001 Median PFS (95% CI) per IRF RECIST v1.1, months 6.8 4.3 (5.7 8.3) (4.0 5.6) 0.59 PFS, HR (95% CI) (0.47 0.76) P-value <0.001 CI, confidence interval; HR, hazard ratio; IRF, independent review facility; NE, not evaluable; OS, overall survival; PFS, progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumours Finn RS, et al. N Engl J Med. 2020;382:1894-905 15
IMbrave150 CLINICAL TRIAL EFFICACY RESULTS: SECONDARY ENDPOINTS atezolizumab + bevacizumab (n=326) sorafenib (n=159) Confirmed ORR per IRF RECIST v1.1, % (95% CI) 27.3 11.9 (22.5 32.5) (7.4 18.0) P-value <0.001 atezolizumab + bevacizumab (n=325) sorafenib (n=158) Confirmed ORR per HCC specific mRECIST, % (95% CI) 33.2 13.3 (28.1 38.6) (8.4 19.6) P-value <0.001 CI, confidence interval; HCC, hepatocellular carcinoma; IRF, independent review facility; mRECIST, modified RECIST; ORR, objective response rate; RECIST, Response Evaluation Criteria in Solid Tumours Finn RS, et al. N Engl J Med. 2020;382:1894-905 16
IMbrave150 CLINICAL TRIAL SAFETY RESULTS atezolizumab + bevacizumab (n=329) sorafenib (n=156) Variables, n (%) Patients with an AE from any cause 323 (98.2) 154 (98.7) Grade 3-4 AEs (numbers represents the highest grades assigned) 186 (56.5) 86 (55.1) Grade 5 AEs 15* (4.6) 9** (5.8) Serious adverse event 125 (38.0) 48 (30.8) AEs leading to withdrawal from any trial drug 51 (15.5) 16 (10.3) AEs leading to dose modification or interruption of any trial drug 163 (49.5) 95 (60.9) Dose interruption of any trial treatment 163 (49.5) 64 (41.0) Dose modification of sorafenib 58 (37.2) *Grade 5 events in the atezolizumab bevacizumab group: gastrointestinal haemorrhage (in 3 patients), pneumonia (in 2 patients), empyema, gastric ulcer perforation, abnormal hepatic function, liver injury, multiple-organ dysfunction syndrome, oesophageal varices haemorrhage, subarachnoid haemorrhage, respiratory distress, sepsis, and cardiac arrest (in 1 patient each) **Grade 5 events in the sorafenib group: death (in 2 patients), hepatic cirrhosis (in 2 patients), cardiac arrest, cardiac failure, general physical health deterioration, hepatitis E, and peritoneal haemorrhage (in 1 patient each) AEs, adverse events Finn RS, et al. N Engl J Med. 2020;382:1894-905 17
IMbrave150 CLINICAL TRIAL CONCLUSION IMbrave150 demonstrated a statistically significant improvement in OS and PFS with atezolizumab + bevacizumab versus sorafenib in the first-line setting in patients with advanced HCC atezolizumab + bevacizumab: approved by US FDA on 29 May 2020 as first-line systemic therapy for advanced HCC Times to response were similar in the combination and sorafenib arms Response rates were significantly higher in the combination arm The trial was conducted in a patient population that had preserved liver function (Child Pugh class A) and a decreased risk of variceal bleeding. The safety of the combination in a broader population warrants further study FDA, Food and Drug Administration; HCC; hepatocellular carcinoma; OS, overall survival; PFS; progression-free survival Finn RS, et al. N Engl J Med. 2020;382:1894-905 18
COR2ED Bodenackerstrasse 17 4103 Bottmingen SWITZERLAND Dr. Froukje Sosef MD +31 6 2324 3636 froukje.sosef@cor2ed.com Dr. Antoine Lacombe Pharm D, MBA +41 79 529 42 79 antoine.lacombe@cor2ed.com Heading to the heart of Independent Medical Education Since 2012