Overview of Mycobacterium and Staining Techniques in the Lab
Mycobacterium is a bacterium known for causing diseases like TB, with unique morphology and staining characteristics. Learn about the various species, specimen sources, morphology, and staining techniques like Ziehl-Neelsen in the lab.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
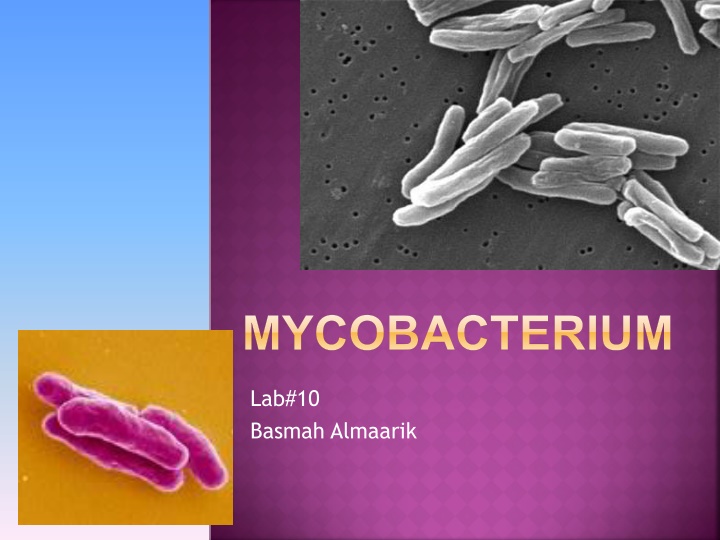
MYCOBACTERIUM Lab#10 Basmah Almaarik
MYCOBACTERIUM It has many species one of them is Mycobacterium tuberculosis that cause TB. Some other common species seen include: M. avium AIDS patients. M. kansasii M. fortuitum
MYCOBACTERIUM Specimen seen in lab are mainly sputum. Bone biopsy, CSF, urine and other.
MYCOBACTERIUM MORPHOLOGY Non spore forming, non capsulated bacilli. It has a fatty cell wall so it dose not stain well with gram stain. The gram stain can not penetrate this layer. Acid Fast Bacilli cause it can not be decolorized with acid.
MYCOBACTERIUM STAINING Ziehl-Neelsen stain. 1. Kinyoun stain (cold method) 2. Fluorochrome stain. 3.
MYCOBACTERIUM STAINING Ziehl-Neelsen technique: Carbolfuchsin Acid alcohol 3% Methylene blue Once Carbolfuchsin go inside the bacterial cell it can not be decolorized by acid because of the fatty cell wall
ZIEHL-NEELSEN TECHNIQUE Do a regular smear. 1. Need longer fixation time because of the thick fatty cell wall Heat fix slide. 2. Cover slide with carbol 3. fuchsin allow heated Stain to stay for 5 min a) We apply heat under slide until vapor just begins to rise (this will allow penetration of stain inside the cells). b) Or we better use heated carbolfuschin and if it dray we apply more of the stain (this to avoid fire hazard)
ZIEHL-NEELSEN TECHNIQUE Wash with water. Now the carbolfoschin and the fatty layer of cell wall make a complex that is red and cannot be decolorized Cover slide with acid alcohol 3-5 mint tell all the red color is gone. (take care cause acid alcohol is flammable) Wash slide well with water. Cover slide with methylene blue for 3-5 mint Wash and examin. 4. 5. 6. 7. 8.
ZIEHL-NEELSEN TECHNIQUE 1 2 3 4 5 6
ZIEHL-NEELSEN TECHNIQUE Results Red bacilli against a blue background 7 Very slender bacilli, arranged in single, or in pairs or in long parallel bundles making cord formation
MODIFIFIED ZIEHL-NEELSEN TECHNIQUE Acid Fast Bacilli Non- Acid Fast Bacilli Carbolfuschin Decolorizer Methylin Blue
KINYOUN STAIN (COLD METHOD) Same steps as Zhiehl-Neelsen but with few changes: No heating Concentrated carbolfuschin. Concentrated decolorizing agent. Same results (red bacilli- blue back ground)
FLUOROCHROME STAIN When exposed to UV light it will fluorescent. These slides are examined using fluorescent microscope. If Bacilli are present it will fluorescence against a dark background. This method is used for screening if positive it is confirmed by Common fluorochrome stain: Auramine-Rhodamin stain Acridine orange stain
FLUOROCHROME STAIN 2. Flood with auramine-phenol stain 10 m 3. Wash with water 1. Heat fix slide 6. Cover with potassium permanganate 10 sec 4. Decolorize 1% acid alcohol 5 m 5. Wash with water
FLUOROCHROME STAIN 7. Rinse thoroughly with water Examine at x25 or X40 Bacilli will appear yellow orange against a black background. Always include a positive control smear with each batch to make sure stain is ok
EXAMINING THE SLIDE Ziehl-Neelsen and Kinoyoun are examined in oil immersion X100 objective Fluorochrome Stine are examined at X20 or X40 objective
MYCOBACTERIUM CULTURE The media used is Lowenstein Jensen (LJ) It is enriched medium Mycobacterium grow aerobically. 35-37 C. Slow grower. M.tuberculosis raised , dry cream (buff) colonies. Visible colonies appear after 2-3 weeks of incubation. But culture should be incubated up to 6 weeks before discarding.
LOWENSTEIN JENSEN Media contain: Glycerol (enhances the growth of Mycobacterium tuberculosis) Antibiotic (Low levels of penicillin and nalidixic acid are also present in LJ medium to inhibit growth of gram positive and gram negative bacteria) Malachite green (inhibits most other bacteria) Egg yolk
LOWENSTEIN JENSEN Coli flower appearance colonies, easily removed but very difficult to emulsify, non pigmented
RAPID METHODS FOR DETECTION BACTEC 12B SYSTEM Fluid medium containing palmotic acid carbon 14 labelled (C14)