Overview of National GRAIL Pilot for Wessex Cancer Alliance
This paper outlines the national expectation for 13 cancer alliances to participate in the NHS-Galleri trial pilot. It summarizes the proposed approach for Dorset and HIOW ICBs, highlighting risks, planning positions, and implications across various domains. Recommendations include supporting the pace of planning, alignment with ICB governance, addressing financial pressures, and embracing the national opportunity for the pilot.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
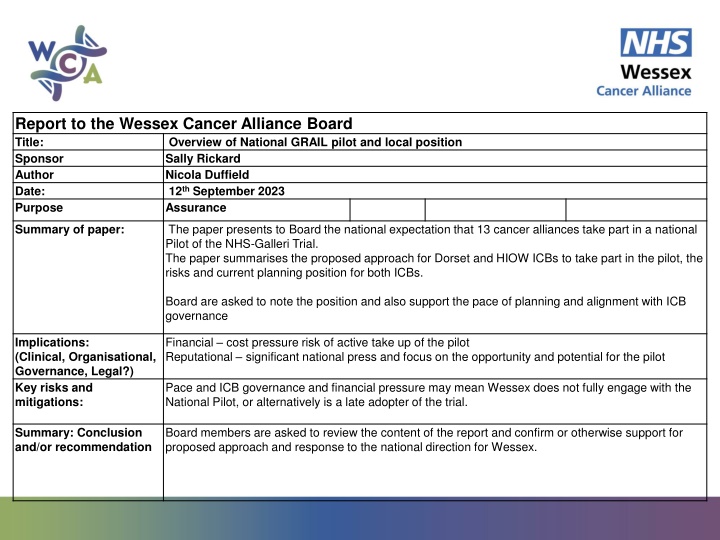
Report to the Wessex Cancer Alliance Board Title: Overview of National GRAIL pilot and local position Sponsor Sally Rickard Author Nicola Duffield Date: 12th September 2023 Purpose Assurance The paper presents to Board the national expectation that 13 cancer alliances take part in a national Pilot of the NHS-Galleri Trial. The paper summarises the proposed approach for Dorset and HIOW ICBs to take part in the pilot, the risks and current planning position for both ICBs. Summary of paper: Board are asked to note the position and also support the pace of planning and alignment with ICB governance Financial cost pressure risk of active take up of the pilot Reputational significant national press and focus on the opportunity and potential for the pilot Implications: (Clinical, Organisational, Governance, Legal?) Key risks and mitigations: Pace and ICB governance and financial pressure may mean Wessex does not fully engage with the National Pilot, or alternatively is a late adopter of the trial. Board members are asked to review the content of the report and confirm or otherwise support for proposed approach and response to the national direction for Wessex. Summary: Conclusion and/or recommendation
National Guidance Deliverable: For Alliances participating in the clinical trail Support retention & onward referral of patients in the NHS-Galleri Clinical trial. For Alliances NOT participating in the clinical trial Establish and test the clinical and operational processes for the GRAIL Interim Implementation Pilot Commission the local biosampling service for the GRAIL Interim Implementation Pilot. 5. Nationally commissioned programmes delivered through Cancer Alliances Category: Rationale: Ensuring sufficient sample size in the NHS-Galleri trial and appropriate onward referral for those with suspected cancer. The NHS-Galleri trial will report on its interim results in March 2024, to demonstrate the effectiveness of this approach as an early diagnosis cancer screening tool. If it meets specific success criteria, then the NHS is committed to rolling out the test at scale in 24/25 and 25/26 in an Interim Implementation Pilot in areas not currently participating in the trial. This work is needed to ensure the system is ready for this roll out. If the Galleri trial is successful, then the screening test will have a significant impact in diagnosing more people earlier. Cancer Alliance role: Supporting GRAIL with comms for recruitment and retention to the trial. Identification of GRAIL Interim Implementation Pilot operational lead and clinical champion to support system oversight and project development by end of Q1. Ensure establishment of onward referral pathway for those with suspected cancer through existing NSS pathway by the end of Q4. Development of communications plan (using national communications materials) by the end of Q3 . Establish and develop a biosampling commissioning approach in collaboration with local ICB's by the end of Q4 that will be ready for 24/25 Q2 implementation. The proposed delivery model for the Interim Implementation Pilot is to use local phlebotomy services. These services will need to be stood up as soon agreement has been reached to process with the Interim Implementation Pilot (decision is due in March 2024 based on interim trial results). Alliances are expected to work with their local ICBs to establish this service, using a national specification and funding that will be provided by the national team. Work with local providers receiving referrals to ensure clinical pathways for onward referral of positive tests are functioning well. Transition to BAU: Cancer Alliances to continue to support the trial until completion. The interim implementation pilot will run for two years. Further roll-out of Galleri testing will be dependent on consideration of the full trial evidence from the UK NSC. Health Inequalities: Prioritising recruitment and communication of the GRAIL clinical trials in more deprived areas (where relevant). Alliances should assess the impact of the interim implementation pilot on health inequalities (e.g., via EHIA), and as part of the comms plan, prioritise participant uptake rates in areas of high deprivation and underserved groups Local areas should be supported in looking at how the biosampling locations can support access to underserved groups, ensuring proportionate take up within groups. Success measures: No specific measures will be set by the national team, activities can be reported as part of the narrative section in quarterly returns. Use of funds (Targeted) Programme management. Any required costs to establish commissioning of services, set up of teams, establishment of processes. Relevant guidance Ongoing monitoring and support GRAIL communications working group folder on Futures Futures folder containing clinical and public facing resources relating to the NHS-Galleri trial Demonstration events targeted at Cancer Alliances/patient groups or clinicians each quarter Monthly meetings for named GRAIL leads Meetings with Communication Leads can be incorporated into Alliance Comms meeting
Overview Context The NHS-Galleri pilot is a large-scale in-service evaluation of a multi-cancer early detection blood test in the NHS. This test has been shown by studies to be effective at finding cancers that are typically difficult to identify early- such as head and neck, bowel, lung, ovarian and pancreatic cancers. For each referral where there is a positive cancer signal is detected an estimated 50% of the patients will have a confirmed cancer diagnosis and 50% will have a false positive (the individual does not have cancer). National plan NHS England has agreed to purchase 1,000,000 Galleri tests over 2 years that will be offered to asymptomatic participants aged 50- 77 years. All 13 cancer alliances that were not involved in the initial trial have been invited to participate in the NHS-Galleri pilot. Each ICB is required to respond to the national team and advise whether they have agreed to host the NHS-Galleri pilot at an executive level. Cost and capacity Blood testing will be fully funded through the NHS England National Cancer Programme. An agreed price of up to 8.50 per blood draw has been agreed by the national team. There is no agreement to support with funding for investigation of patients referred with a positive cancer signal. From initial trial data the likely impact is that 92% of patients with a cancer signal detected will undergo an imaging procedure, such as CT scan or MRI. Most of the diagnosed participants (82%) will undergo an invasive procedure to confirm the diagnosis. A smaller proportion of the false positives will undergo an invasive procedure (30%).
Dorset Activity Expected activity for duration of pilot (2 years) Eligible population for Galleri testing 195,000 Asymptomatic population aged 50-77 years Galleri blood tests 19,500 Based on trial data, expected uptake rate of 10% 2ww referrals 195 Based on trial data, estimated 1% will have a positive cancer signal detected and so require onward referral Additional cancers diagnosed 98 For each referral where there is a positive cancer signal is detected an estimated 50% of the patients will have a confirmed cancer diagnosis and 50% will have a false positive (the individual does not have cancer). Activity Unit cost Annual activity Cost per year 165,750 Blood tests 8.50 19,500 16,268 2ww referral 166+MFF 98 17,640 Imaging 196+MFF 90 14,355 Invasive diagnostic procedure 261+MFF 55 214,013 Estimated annual cost
HIOW Activity Expected activity for duration of pilot (2 years) Eligible population for Galleri testing 640,000 Asymptomatic population aged 50-77 years Galleri blood tests 64,000 Based on trial data, expected uptake rate of 10% 2ww referrals 640 Based on trial data, estimated 1% will have a positive cancer signal detected and so require onward referral Additional cancers diagnosed 320 For each referral where there is a positive cancer signal is detected an estimated 50% of the patients will have a confirmed cancer diagnosis and 50% will have a false positive (the individual does not have cancer). Activity Unit cost Annual activity Cost per year 544,000 Blood tests 8.50 64,000 53,120 2ww referral 166+MFF 320 57,624 Imaging 196+MFF 294 46,719 Invasive diagnostic procedure 261+MFF 179 701,463 Estimated annual cost
Key points to note: National planning sets out a time frame for implementation for the pilot phase from Phase 1 (Q2 24/25) to Phase 3 (Q4 24/25 to Q4 25/26). Wessex Cancer Alliance is planning to be part of the Phase 3 Main Phase . Participants will be informed of the test result by letter. Participants that receive a Cancer Signal Detected result will be informed of this outcome by a senior clinician (likely band 7 nurse or higher), in National Operations Service Clinical Team These participants will then be referred to the local non-specific symptoms (NSS) pathway for diagnostic follow up. For both HIOW and Dorset decision is needed if existing NSS (RIS) service will be support the Galleri Pilot Transformation funding will be available via the Wessex Cancer Alliance to support Dorset and HIOW recognising anticipated pressures with diagnostic investigation as a result of additional two-week wait referrals from the pilot. No data are available on the likelihood of incidental findings during diagnostic workup following a Cancer Signal Detected Galleri test result. The test is designed to detect cancer and is not recommended to support the detection or diagnosis of other diseases. Learning from Targeted Lung Health Checks however suggests significant incidental findings should be anticipated following scanning of an asymptomatic population. WCA plan to review data locally from RIS and TLHC to estimate anticipated incidence of incidental findings. WCA, in partnership with ICS representatives must develop a detailed communications approach, targeting populations that may be vulnerable and benefit from proactive early engagement or support. ICBs can direct the national team invitations to PCNs.