Oxidative Addition and Related Mechanisms in Inorganic Chemistry
Explore the mechanisms of oxidative addition, reductive elimination, migratory insertion, and CO insertion reactions in inorganic chemistry. Learn how metal complexes undergo bonding transformations by breaking and forming bonds with anionic ligands. Discover the key concepts and stereochemistry involved in these reactions.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
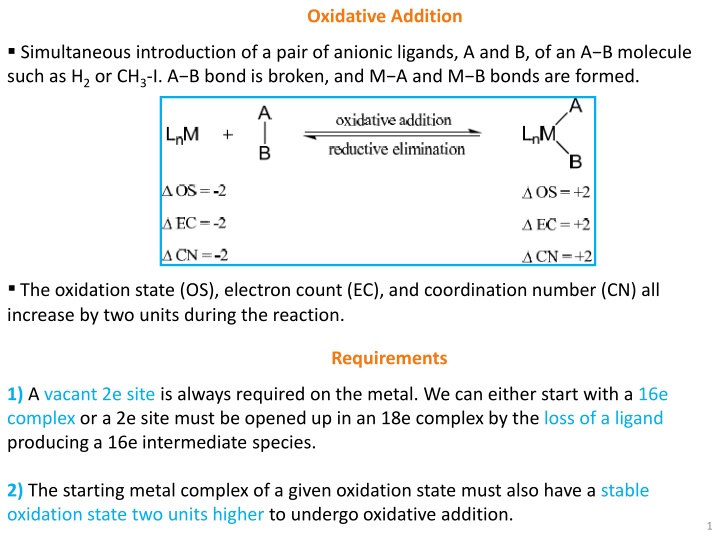
Oxidative Addition Simultaneous introduction of a pair of anionic ligands, A and B, of an A B molecule such as H2 or CH3 I. A B bond is broken, and M A and M B bonds are formed. The oxidation state (OS), electron count (EC), and coordination number (CN) all increase by two units during the reaction. Requirements 1) Avacant 2e siteis always required on the metal. We can either start with a 16e complex or a 2e site must be opened up in an 18e complex by the loss of a ligand producing a 16e intermediate species. 2) The starting metal complex of a given oxidation state must also have a stable oxidation state two units higherto undergo oxidative addition. 1
Oxidative Addition to Vaskas Complex HX Cl X 2
Overview of Oxidative Addition Mechanism Type of A-B Stereochemistry Concerted Fairly non-polar substrates: H-H, R3C-H, R3Si-H Polarized substrates: R3C-X Also Cl2, Br2, I2 R3C-X, R3Sn-X H-X (largely dissociated in solution) cis-addition SN2 trans-addition Radical - Ionic - Non-polar substrates (e.g. H-H, C-H, Si-H) Concerted Alkyl halides Nucleophilic (SN2) or Radical Halogens (Cl2, Br2, I2) Nucleophilic (SN2) Acids (HCl, HBr, HI) Ionic 3
Concerted Mechanism Two-Step Mechanism: 1) Incoming ligand first binds as a complex, 2)Bond breaking as a result of strong back donation from metal into the * orbital. SN2Mechanism (Non Concerted) The metal electron pair of LnM directly attacks the A B * orbital at the least electronegative atom. 4
Reductive Elimination Reductive elimination, the reverse of oxidative addition, is most often seen in higher oxidation states because the formal oxidation state of the metal is reduced by two units in the reaction. 5
Migratory Insertion A migratory insertion reaction occurs when a cisoidal anionic and neutral ligand on a metal complex couple together to generate a new coordinated anionic ligand. 1) 1,1 insertion in which the metal and the X ligand end up bound to the same (1,1) atom. 2) 1,2 insertion in which the metal and the X ligand end up bound to adjacent (1,2) atomsof an L type ligand. A 2e vacant site is generated by insertion reactions. Conversely, elimination requires a 2e vacant site. The insertion requires a cis arrangement of the ligands, while the elimination generates a cis arrangement of these ligands. 6
CO Insertion Reactions 13 13 13 When the incoming ligand is 13CO, the product contains only one labeled CO, which is cis to the newly formed acetyl group. This shows that the methyl group migrates to a coordinated CO, rather than free COattacking the Mn Me bond. We can tell where the labeled CO is located in the product because there is a characteristic shift of the (CO) stretching frequency to lower energy in the IR spectrum of the complex asa result of the greater mass of 13C over normal carbon. 7
Alkene Insertions Reactions 2 ligands like alkenes give 1,2-insertion. This is the reverse of the familiar elimination reaction. Site Selectivity: The site selectivity of 1,2-insertion can be predicted using resonance forms and partial charges. 8
Eliminations Elimination reactions are just the reverse of migratory insertion reactions. -hydride elimination: elimination is the chief decomposition pathway for alkyls that have H substituents. -hydride elimination: If an alkyl has no hydrogens, it may break a C H bond in the , , or position. carbonyl elimination or decarbonylation: 9
