p-Block Elements and Their Characteristics
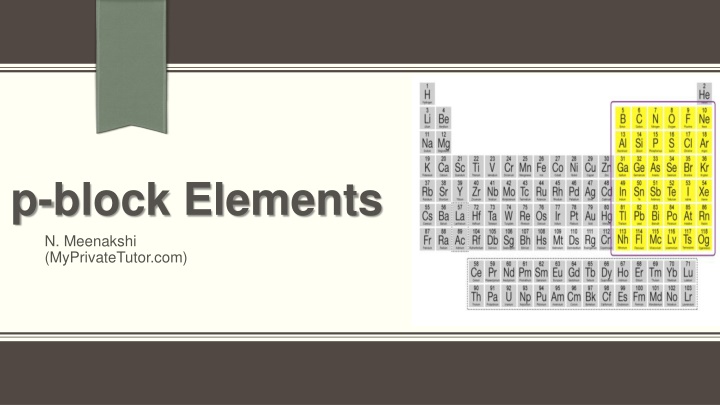
p-Block elements consist of groups 13 to 18 in the periodic table, each group with unique properties. Elements in groups 15 to 18, such as nitrogen, oxygen, halogens, and noble gases, have distinct electronic configurations and behavior. Understanding their valence shell configurations and properties is essential in studying their atomic and ionic radii.
Uploaded on Apr 24, 2025 | 0 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
p-block Elements N. Meenakshi (MyPrivateTutor.com)
Introduction The p-Block elements: Elements belonging to groups 13 to 18 of the periodic table are called p-block elements. General electronic configuration of p-block elements: The p-block elements are characterized by the ns2np1-6valence shell electronic configuration. Representative elements: Elements belonging to the s and p-blocks in the periodic table are called the representative elements or main group elements.
Group 15 Elements or Nitrogen family Group 15 elements are also called Nitrogen family includes nitrogen phosphorus, arsenic, antimony and bismuth elements. Electronic Configuration The valence shell electronic configuration plays a major role in how valence electron an element behaves. The valence electron shell configuration of group 15 elements is ns2np3. All the group 15 elements have the same arrangement and this is why they re similar. The s-orbital in this group is completely filled and the p-orbitals are half filled and this makes their configuration extra stable.
Group 16 Elements or Oxygen family Group 16 elements are also known as Oxygen family and includes oxygen, sulphur, selenium, tellurium, and polonium. Electronic configuration The elements of group 16 have a valence shell electronic configuration of ns2np4.
Group 17 Elements or Halogen group Group 17 elements are also known as Halogens and includes fluorine, chlorine, bromine, iodine and astatine. Electronic configuration The elements of group 17 have a valence shell electronic configuration of ns2np5.
Group 18 Elements or Noble gases Group 17 elements are also known as Noble gases and includes helium, neon, argon, krypton, xenon, radon. Electronic configuration The elements of group 18 have a valence shell electronic configuration of ns2np6. These gases are chemically unreactive. They form very few compounds, because of this they are termed as noble gases . Xenon and radon are the rarest elements of the group.
Atomic and Ionic Radii If you see the electronic configuration of elements in the table, you will notice that with every step you move downwards, new orbitals are added to the atom. This addition of new orbitals increases both the Atomic and the Ionic radii of group 15 elements. However, we see that from Arsenic to Bismuth only a small increase in ionic radius is observed. This is due to the presence of completely filled d and/or f orbitals in heavier members
Ionization Enthalpy Ionization Energy is the amount of energy required to remove an electron from the outermost orbit of the atom. This is basically a measure of how hard the nucleus is holding on to the electron. The closer the electron is to the nucleus the stronger its hold and thus the energy required is more. As we move down the group, the radius of the atom increases and therefore the Ionization energy decreases due to the weaker hold of the nucleus. Due to stable electronic configuration noble gases exhibit very high ionisation enthalpy. Electronegativity The tendency or the ability of an atom/ion/molecule to pull e-s towards itself. The electronegativity value decreases down the group with increasing atomic size. This again is due to the increasing distance between the nucleus and the valence shell as we move down the group.
Electron gain enthalpy: the amount of energy released when an electron is added to an isolated gaseous atom. Due its small size, oxygen has negative value. However, the value becomes less negative ( or positive) as we go down the group. Halogens have maximum negative values, and the value becomes less negative ( or positive) as we go down the group. Fluorine has less negative value than chlorine, due to its small size. This results interelectronic repulsion in 2p orbital of fluorine. Since noble gases have stable electronic configurations, they have no tendency to accept the electron and therefore, have large positive values of electron gain enthalpy. Metallic character: due to low ionization enthalpy values, the metallic character increases down the group. Non-metallic character: due to high ionization enthalpy values, halogens are non-metals. Non-metallic character decreases down the group.
Physical Properties group 15 All the elements of the group exist in a polyatomic state. The first, Nitrogen is gas but as you move down there is a significant increase in the metallic character of the elements. Nitrogen and Phosphorus are non-metals, Arsenic and Antimony are metalloids and Bismuth is a metal. These changes can be attributed to the decrease in Ionization enthalpy and increase in atomic size. Boiling points also, in general, show an increasing trend as you move down. Except for Nitrogen, all the other elements have allotropes.
Physical properties group 16 Oxygen is a gas. Oxygen and sulphur non-metals; selenium and tellurium metalloids, polonium metal Polonium a radioactive metal and is short-lived (half life is approximately 14 days) All the elements in group 16 exhibit allotropy. O2 diatomic molecule and other elements of the group are generally polyatomic like sulphur S8 Boiling and melting points of these elements are high (due to polyatomicity)
Physical properties group 17 Fluorine and chlorine gases, bromine liquid, iodine solid. Astatine radioactive element. Boiling point and melting point - very high and it increases down the group. Bond dissociation enthalpy of F2is smaller than Cl2, due to its small size (interelectronic repulsion). From chlorine, the value of dissociation decreases. Colour: all halogens are coloured. This is due to absorption of radiations in visible region which results in the excitation of outer electrons to higher energy level. By absorbing different quanta of radiation, they display different colours.
Physical properties group 18 All elements are monoatomic. Colourless and odourless Sparingly soluble in water. Low boiling and melting points presence of weak dispersion forces (Helium bp = 4.2 K)
Chemical Properties Oxidation state The valence shells of the p-Block elements have a configuration of ns2np3. So the elements here can either lose 5 electrons or gain 3. The common oxidation states of these elements are -3, +3 and +5. With a decrease in the Ionization enthalpy and electronegativity, due to the increasing atomic radius, the tendency to gain three electrons to create a -3 oxidation state decreases down the group. In fact, Bismuth hardly forms any compounds with -3 oxidation state. As we go down, the stability of the +5 state decreases and that of +3 increases due to inert pair effect. Inert pair effect: The tendency of ns2 electron pair to participate in bond formation decreases with the increase in atomic size. Within a group the higher oxidation state becomes less stable with respect to the lower oxidation state as the atomic number increases. This trend is called inert pair effect . In other words, the energy required to unpair the electrons is more than energy released in the formation of two additional bonds.
Chemical properties Oxidation state Electronegativity of oxygen is very high, it shows only negative oxidation state as 2 except in the case of OF2 where its oxidation state is + 2. The stability of -2 oxidation state decreases down the group. The stability of + 6 oxidation state decreases down the group and stability of + 4 oxidation state increases (inert pair effect).
Oxidizing nature of halogens: F2 is the strongest oxidising halogen and it oxidises other halide ions. F2 + 2X 2F + X2 (X = Cl, Br or I) The oxidizing ability of other halide ions decreases down the group. This is evident from their standard electrode potentials.
The relative oxidising power of halogens can further be illustrated by their reactions with water
Anomalous Properties of Nitrogen Nitrogen, the first element from group 15, differs from others group members because of :- i) Small size of N atom. ii) High estimation of electro-negativity of N atom and high ionization energy. iii) Absence of d-orbitals in the outermost electron shell. iv) Tendency of forming multiple bonds (p -p ) with itself and with other elements having small size and high electronegativity.
Anomalous Properties of Nitrogen Phosphorus, arsenic and antimony form single bonds as P P, As As and Sb Sb while bismuth forms metallic bonds in elemental state. N N bond is weaker than the single P P bond because of high interelectronic repulsion of the non- bonding electrons. Therefore, catenation property is weaker in case of nitrogen. Nitrogen cannot form d p bond as the heavier elements can e.g., R3P = O or R3P = CH2 (R = alkyl group). Phosphorus and arsenic can form d d bond.
Chemical Properties Reactions 1) Reactivity with hydrogen EH3 (E = element and H = hydrogen) Reactivity with oxygen - E2O3and E2O5 2) 3) Reactivity with halogens - EX3and EX5 4) Reactivity with metals forms binary compounds exhibiting 3 oxidation state
Oxides of Nitrogen
Anomalous Properties of Oxygen Oxygen being small in size, highly electronegative, and non-availability of valence electrons in d orbital oxygen shows anomalous behavior. Oxygen is diatomic whereas others are polyatomic. Oxygen is a gas while others are solid. Oxygen shows negative oxidation states except in OF2 exhibits H-bonding, as seen in case of H2O
Chemical Properties Reactions 1) Reactivity with hydrogen H2E (E = element and H = hydrogen) Reactivity with oxygen EO2and EO3 2) 3) Reactivity with halogens EX2, EX4and EX6
Anomalous Properties of Fluorine Anomalous behaviour of fluorine is due to its small size, highest electronegativity, low F-F bond dissociation enthalpy, and non availability of d orbitals. Ionisation enthalpy, electronegativity, and electrode potentials are all higher. Ionic and covalent radii, m.p. and b.p., enthalpy of bond dissociation and electron gain enthalpy are quite lower. All reactions are exothermic. forms only one oxoacid while other halogens form a number of oxoacids. Hydrogen fluoride is a liquid due to strong hydrogen bonding.
Chemical Properties Reactions 1) Reactivity with hydrogen Hydrogen halides (H-X) (X = any halogen and H = hydrogen) 2) Reactivity with oxygen Oxides (range of oxides are formed) 3) Reactivity with halogens Interhalogen compounds XX, XX3, XX5, XX7 Reactivity with metals Metal halides (M-X) (X = any halogen and M = metal) 4)
Chemical properties group 18 Noble gases are inert or non-reactive for the following reasons: The noble gases except helium (1s2 ) have completely filled ns2 np6 electronic configuration in their valence shell. (i) (ii) They have high ionization enthalpy and more positive electron gain enthalpy. In 1962, a person named, Neil, prepared the first compound of noble gas Xe+ PtF6 This compound was prepared by mixing PtF6 and Xe. .