Palladium-Catalyzed Couplings Literature Examples
Explore examples of palladium-catalyzed couplings including Heck and Sonogashira couplings for alkenes and alkynes, as well as Negishi, Stille, Suzuki-Miyaura, and Hiyama couplings using various elements. Learn about specific reaction details and applications in organic synthesis.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Palladium-catalyzed couplings: Literature examples Heck and Sonogashira couplings (alkenes & alkynes) Negishi, Stille, Suzuki-Miyaura, Hiyama (Zn, Sn, B, Si) Martin A. Walker, SUNY Potsdam CC-BY-SA 3.0 license
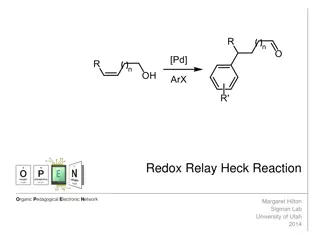
alkene Simple Heck coupling Heck, R. F. Org. React. 1982, 27, 345.
alkene An intramolecular Heck coupling Heck coupling to a trisubstituted alkene is difficult, hence a more active trifluoroacetate catalyst is used. Note also how the double bond migrates ; this quite often occurs. Hong, C. Y.; Kado, N.; Overman, L. E. J. Am. Chem. Soc. 1993, 115, 11028-11029.
alkyne The Sonogashira coupling Use of the Sonogashira coupling to couple an alkyne with a vinyl iodide. The product (after removal of the TMS group) is (+)-obtusenyne, a metabolite of Laurencia red algae. Crimmins MT, Powell MT. " Enantioselective Total Synthesis of (+)-Obtusenyne", Journal of the American Chemical Society, 2003, 125 (25), 7592 7595. doi:10.1021/ja029956v
organozinc Negishi coupling dppe is a bidentate phosphine ligand Herbert, John M. Tetrahedron Lett. 2004, 45(4), 817 819.
organotin Stille coupling: Preparation of an aryl triflate, followed by a Stille coupling. Furstner A, Seidel G, Kindler N. "Macrocydes by Ring-Closing-Metathesis, XI 1: Syntheses of (R)-(+)-Lasiodiplodin, Zeranoi and Truncated Salicylihalamides", Tetrahedron1999, 55, 8215-8230. doi:10.1016/S0040-4020(99)00302-6
organoboron The Suzuki-Miyaura reaction The arylboronic acids & esters are often prepared from aryllithiums. Denton TT, Zhang X, Cashman JR. "5-Substituted, 6-Substituted, and Unsubstituted 3-Heteroaromatic Pyridine Analogues of Nicotine as Selective Inhibitors of Cytochrome P-450 2A6", Journal of Medicinal Chemistry, 2005, 48 (1), 224 239. doi:10.1021/jm049696n
organoboron Suzuki-Miyaura coupling in synthesis A typical use of the Suzuki-Miyaura coupling in synthesis is shown in Mandal's 2002 synthesis of (-)-ebelactone A. Note that here an alkylborane is used, easily prepared by hydroboration. dppf is a bidentate phosphine ligand. Mandal, AK. "Stereocontrolled Total Synthesis of (-)-Ebelactone A", Organic Letters, 2002, 4, 2043-2045. doi:10.1021/ol020058d
organoboron Molander variation of the Suzuki-Miyaura coupling This uses an alkyltrifluoroborate salt. Molander GA, Petrillo DE. "Suzuki-Miyaura Cross-Coupling of Potassium Trifluoroboratohomoenolates", Organic Letters, 2008, 10, 1795-1798. doi:10.1021/ol800357c
organosilane Hiyama coupling Pierrat P, Gros P, Fort Y. "Hiyama Cross-Coupling of Chloro-, Fluoro-, and Methoxypyridyltrimethylsilanes: Room-Temperature Novel Access to Functional Bi(het)aryl", Organic Letters, 2005, 7(4), 697-700. doi:10.1021/ol047482u