Patient ESA Type Distribution in European Countries 2010-2015
Explore the distribution of ESA types among patients in Belgium, Germany, Italy, Spain, Sweden, and the UK from 2010 to 2015. Gain insights into the prescription trends for ESA-treated patients and the use of different forms of ESA.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
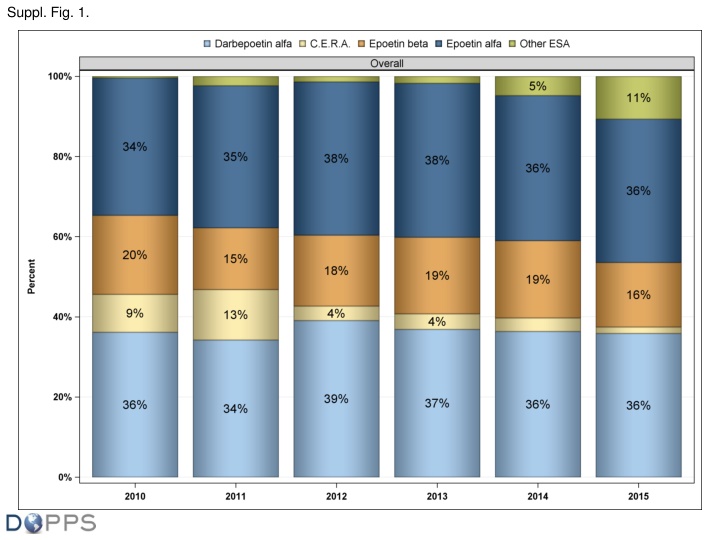
Suppl. Fig. 1. Patient ESA type distribution (Belgium, Germany, Italy, Spain, Sweden, UK), 2010 to 2015. Values for each month reflect prescription among ESA-treated patients. Other ESA refers to non- / epoetin forms, primarily epoetin . Epo- includes biosimilars regulated by the European Medicines Agency and other copies not authorized by the European Medicines Agency. Abbreviations: CERA, continuous erythropoietin receptor agonist (methoxy polyethylene glycol-epoetin ).
Suppl. Fig. 2. Patient ESA type distribution by country, 2010 to 2015. Values for each month reflect prescription among ESA-treated patients. Other ESA refers to non- / epoetin forms, primarily epoetin . Epo- includes biosimilars regulated by the European Medicines Agency and other copies not authorized by the European Medicines Agency. Abbreviations: CERA, continuous erythropoietin receptor agonist (methoxy polyethylene glycol-epoetin ).
