pH and Indicators in Chemistry
Explore the concept of pH and indicators in chemistry to distinguish between acids and bases, understand pH scales, and safely test pH levels using indicators. Learn how indicators change color based on the pH of a solution and practice using indicators to determine pH ranges. Discover the properties of neutral, acidic, and basic solutions through engaging visuals and practical examples.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
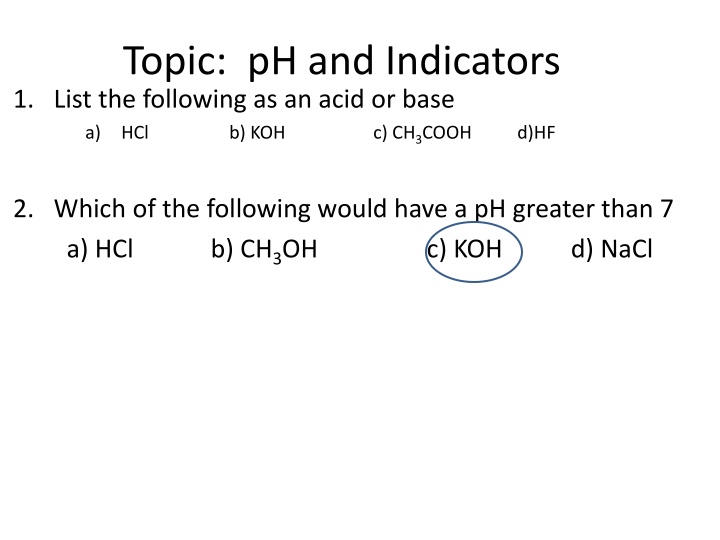
Topic: pH and Indicators 1. List the following as an acid or base a) HCl b) KOH c) CH3COOH d)HF 2. Which of the following would have a pH greater than 7 a) HCl b) CH3OH c) KOH d) NaCl
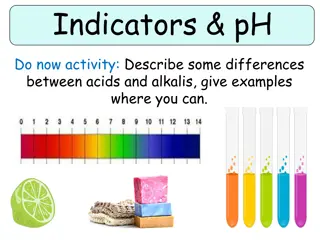
pH scale power (or potential) of Hydrogen Ranges from 0-14 measures H+concentration [H+] the more H+, the more acidic the solution
Acid, Base, or Neutral Neutral solution: pH = 7 [H+] = [OH-] H+1 > OH-1 OH-1 > H+1 Acidic solution: pH LESS THEN 7 Basic solution: pH GREATER THEN 7
How to safely test pH NEVER taste Instruments use a pH meter See if the substance reacts with a metal other than Cu, Ag, or Au Indicators use a series of indicators
Indicator substance that changes color over narrow pH range
What color would the following indicators be in a neutral solution? yellow green colorless purple blue yellow
Use several indicators to narrow down pH range of substance Ex: Three samples of the same solution are tested, each with a different indicator. All three indicators, bromthymol blue, bromcresol green and thymol blue, appear blue The pH of the solution must be greater than_____
Practice using table M 1. What indicator is yellow with a pH 9.8 2. Which indictor is blue with a pH of 5.6 0-3.0 4.5-14 3.1.-4.4 Orange Green Pink 7.7-14 8.4-14 Purple 3.8-5.4 0-3.7 5.5-14 Green 9.7-14 Green