Phase Changes in Matter
This content explains phase changes in matter using a phase change graph and diagrams. It discusses plateaus in temperature during melting, freezing, evaporation, and condensation, highlighting when kinetic energy is added or removed in different phases.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
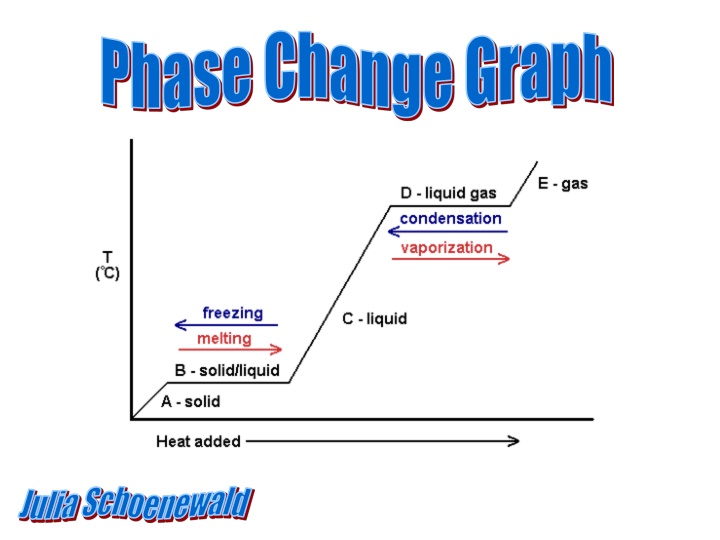
Phase Change Graph Julia Schoenewald
Explanation: page 403 Picture: page 523 Kinetic Energy is added to a liquid causing the temperature to rise Eventually a phase change occurs and you observe a plateau During a phase change the temperature remains constant Phase changes include melting, freezing, evaporation, and condensation
Where does the diagram first plateau and why? What is happening to the kinetic energy in Leg C? Where does the diagram plateau again and why?
Answers It plateaus at 0 C because a phase change is a occurring. Either freezing or melting is happening at this temperature. During a phase change the temperature doesn t change. During Leg C more kinetic energy is being added to the liquid causing the temperature to rise. It plateaus at 100 C because there is a phase change. Evaporation or condensation is occurring at this temperature. During a phase change the temperature doesn t change.
From To Name Energy Leg B (A C) Leg B (C A) Leg D (C E) Leg D (E C)
From To Name Energy Leg B (A C) solid liquid melting rising Leg B (C A) liquid gas evaporation rising Leg B (C E) gas liquid condensation falling Leg B (E C) liquid solid freezing falling