
Pinacol Pinacolone Rearrangement Explained
Discover the fascinating Pinacol-Pinacolone rearrangement process where pinacols are converted into ketones or aldehydes using acids. Learn about the mechanism and industrial uses of glycol. Explore how molecular rearrangement occurs and the applications of pinacolone in various industries.
Uploaded on | 3 Views
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
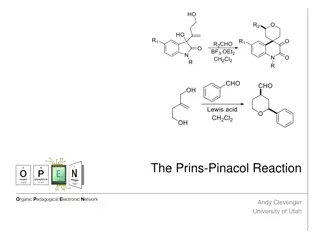
Pinacol- Pinacolone Rearrangement (pinacol rearrangement)
The conversion of pinacols (1,2 glycols) to ketones or aldehydes by means of acids is known as pinacol- rearrangement.
1,2glycolis calledpinacole.When 1,2 glycol is distilled with dilute sulphuric acid, molecular rearrangement takes place to form pinacolone. This rearrangement is a special change in 1,2 glycols.
Mechanism: (i) The basic OH group recievesa proton from sulphuric acid and becomes protonatedglycol.
(ii) This protonatedglycol loses H2O and becomes carbonium ion.
(iii) This carbonium undergoes 1,2 alkyl shift to give more stable carbonium ion.
(iv) This stable carbonium ion loses proton to form pinacolone.
Uses:Glycol has many industrial applications. - It is used as antifreeze in automobile radiators. - It is a solvent and preservative. -It prevents formationof ice on aeroplane wings.
-Dinitrate of glycol mixed with trinitro glycerineis an explosive. -It is used as coolant for aeroengines and as a dielectric in electricalcondensers.













![The Synthesis of Cedranoid Sesquiterpenes via Photo-Rearrangement of Bicyclo[2.2.2] Octenones](/thumb/198279/the-synthesis-of-cedranoid-sesquiterpenes-via-photo-rearrangement-of-bicyclo-2-2-2-octenones.jpg)

