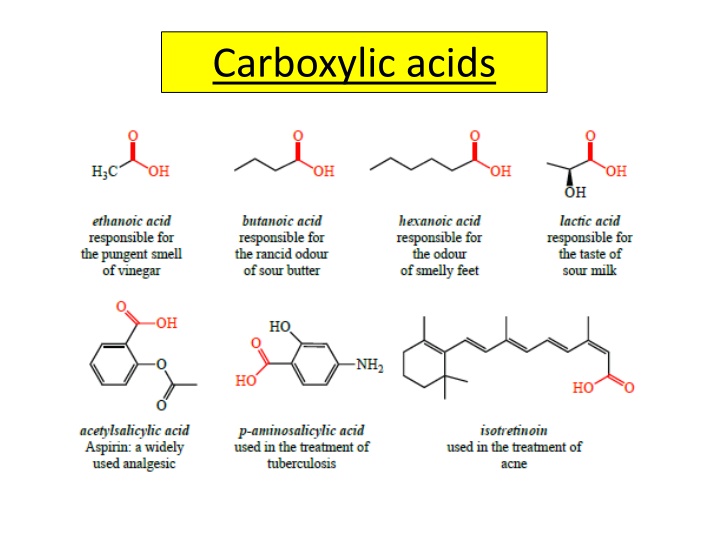
Preparation and Reactions of Carboxylic Acids
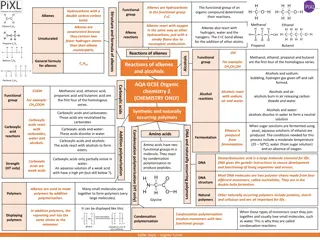
Carboxylic acids are versatile compounds with various preparation methods and reactions. They behave as acids in solutions, forming salts with metals and bases. Through condensation, esters can be synthesized. Additionally, reactions with alcohols yield amides, and they can be reduced to primary alcohols. The compounds are significant in synthesizing useful products.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Preparation of carboxylic acids http://www.durhamcollege.ca/wp-content/uploads/Chemical-Laboratory-Technician-Program-02.jpg
1. oxidation of a primary alcohol
5. Hydrolysis of an ester http://www.chem.ucalgary.ca/courses/350/Carey5th/Ch20/rconr2h2o.gif
Reactions of carboxylic acids Carboxylic acids behave as typical acids in aqueous solution and form salts by reacting with metals and bases(including alkalis). They undergo condensation reactions with alcohols to form esters. Carboxylic acids react with amines to form amides. They can be reduced to primary alcohols using lithium aluminium hydride Carboxylic acids are extremely versatile compounds which can be used to synthesise many useful products.
1. Carboxylic acids and metals Carboxylic acid + metal salt + hydrogen 2CH3COOH (aq) + Mg (s) Mg 2+ (CH3COO-)2(aq) + H2 (g) Magnesium reacts with ethanoic acid to produce magnesium ethanoate and hydrogen gas.
2. Carboxylic acids and bases Carboxylic acid + base salt + water + carbon dioxide CH3COOH(aq) + 2NaOH(aq) 2Na+CH3COO-(aq) + H2O (l) + CO2(g)
4. With amino groups to form amide links
5. Reduction with Lithium aluminium hydride to form primary alcohols.