Preparing Benzocaine for Pain Relief
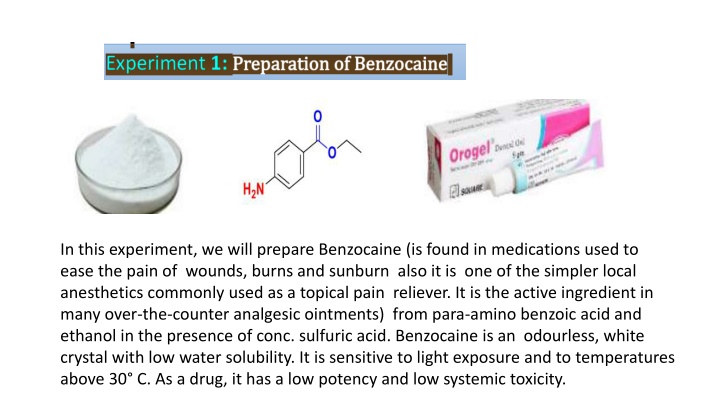
In this experiment, Benzocaine is synthesized from para-amino benzoic acid and ethanol in the presence of sulfuric acid. Changes in its structure affect its pharmacological properties, showcasing how small alterations can impact a molecule's characteristics. The process involves esterification and precipitation steps, leading to the formation of Benzocaine crystals. These crystals are then collected and analyzed for yield percentage. Additionally, the efficacy of Benzocaine as a topical pain reliever and sunscreen ingredient is examined based on its ability to absorb UV radiation. Understanding these chemical transformations is essential for drug development and sun protection research.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
In this experiment, we will prepare Benzocaine (is found in medications used to ease the pain of wounds, burns and sunburn also it is one of the simpler local anesthetics commonly used as a topical pain reliever. It is the active ingredient in many over-the-counter analgesic ointments) from para-amino benzoic acid and ethanol in the presence of conc. sulfuric acid. Benzocaine is an odourless, white crystal with low water solubility. It is sensitive to light exposure and to temperatures above 30 C. As a drug, it has a low potency and low systemic toxicity.
Esterification of p-aminobenzoic acid change its pharmacological properties. This illustrates the point that a relatively small change in structure can dramatically alter the physiological and biological properties of the molecule. Pharmaceutical companies frequently develop a new drug by making small alteration in the structure. The effectiveness of each analogue as a sunscreen will be assessed by measuring how well it absorbs ultraviolet radiation from the sun ( =300nm). Exposure to this radiation can cause sunburn. Prolonged or severe exposure can cause skin cancer and genetic mutation. Sunscreens work because they absorb UV radiation of the appropriate wavelengths at the surface of the skin.
Addition of sulfuric acid causes precipitation of the hydrogen sulfate salt of p-aminobenzoic acid, however this precipitate will dissolve during the reflux period as the hydrogen sulfate salt of the acid is converted into the hydrogen sulfate salt of the ester.
Collect the precipitated crude benzocaine by vacuum filtration. Use additional portions of water to aid in transferring the crystals to the funnel and wash the crystals with water on the funnel. Dry the crystals overnight and calculate the percentage yield for benzocaine.