Process Analysis and Design: Informal Mass Balances Overview
Explore a detailed analysis of a chemical process involving informal mass balances to calculate approximate flow rates and assess process efficiency. The exercise involves determining flow rates in mol/min, designing processes with approximate flow rates, and evaluating mass balances to optimize pure output.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
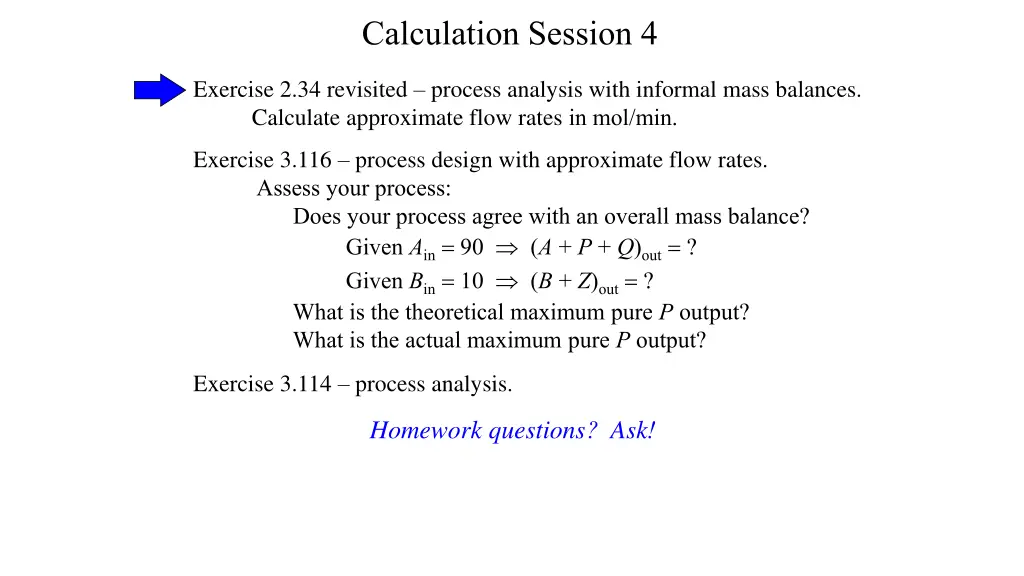
Calculation Session 4 Exercise 2.34 revisited process analysis with informal mass balances. Calculate approximate flow rates in mol/min. Exercise 3.116 process design with approximate flow rates. Assess your process: Does your process agree with an overall mass balance? Given Ain= 90 (A + P + Q)out= ? Given Bin= 10 (B + Z)out= ? What is the theoretical maximum pure P output? What is the actual maximum pure P output? Exercise 3.114 process analysis. Homework questions? Ask!
Calculation Session 4 Exercise 2.34 (Revisited) Created by: Chida Balaji 13 Revised by: Andrew Broenen 13, Alanda Szkodny 14, Kathy Fein 15, Jesse Garcia 16, Jenny Bushnell 17, Joe Hassler 18, Francis Ledesma 19, Michelle Quien 20, Gavin Batsimm 21, Kelsey Levine 22, Leon Lee 23, Burke Combs 24, and Ashlyn Dumaw 25
Goal: Calculate flow rates and reactor/separator capacities using informal mass balances and determine the better design Info: Two proposed design solutions Inlet flow rates for the components Where to start: Trace through provided flow rates Use mass balances to solve the rest
Process A H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) G (3.0 mol/min) water steam What are the main differences? liquid-gas separator 110 C F G B P P F I G H2O F I reactor F + G P I + G B 20 C Which would you guess is a better process? liquid-gas separator 60 C F I P to market F G B F G B F G B B liquid-gas separator 80 C to market F G purge Process B H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) G (3.0 mol/min) water steam liquid-gas separator 110 C F I G B F G B P P Reactions: H2O F I reactor F + G P I + G B 20 C liquid-gas separator 60 C F I F + G P (90% conversion) I + G B (100% conversion) F G B F G B P to market purge: F (0.010 mol/min) G (0.010 mol/min) B
H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) Process A G (3.0 mol/min) water Overall Mass Balance steam liquid-gas separator 110 C F G B P P F I G H2O F I (2.0) reactor F + G P I + G B 20 C (1.0) (2.0) liquid-gas separator 60 C (1.0) F I (2.0) P to market F G B F G B F G B B liquid-gas separator 80 C to market F G purge
Process A: Overall Mass Balance Reactions: F + G P (90% conversion) I + G B (100% conversion) G (3.0) B (2.0) H2O (1000) F (1.0) I (2.0) I does not leave the process All I must be converted to B P (1.0) 90% of the F is converted So 0.9 P leaves the process H2O No F leaves the process All F is converted to P
H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) Process A G (3.0 mol/min) water steam liquid-gas separator 110 C F G B P P (1.0) F I G H2O F I (2.0) (2.0) (2.0) (1.0) reactor F + G P I + G B 20 C (1.0) liquid-gas separator 60 C F I (1.0) (2.0) P to market (1.0) F G B (2.0) F G B (2.0) F G B (2.0) B liquid-gas separator 80 C (2.0) to market F G purge
Process A: Reactor Mass Balance Reactions: F + G P (90% conversion) I + G B (100% conversion) (0.11) (0.11) (1.11) (2.0) (2.0) (3.11) Pout = 0.9*Fin (1.0) 1.0 = 0.9*Fin Fin = 1.11
H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) Process A G (3.0 mol/min) water steam liquid-gas separator 110 C (0.11) (0.11) F G B P P (1.0) (1.1) F I G H2O F I (2.0) (2.0) (1.0) (2.0) (3.1) reactor F + G P I + G B 20 C (1.0) liquid-gas separator 60 C F I (1.0) (2.0) P to market (1.0) (0.11) F G B (0.11) (2.0) F G (0.11) B (2.0) (0.11) F G B (0.11) (2.0) B (0.11) liquid-gas separator 80 C Moles are not conserved Mass is! (2.0) to market F G (0.11) purge (0.11)
Process B H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) G (3.0 mol/min) water steam liquid-gas separator 110 C F I G B F G B P P (2.0) H2O F I reactor F + G P I + G B 20 C (1.0) (2.0) liquid-gas separator 60 C F I (1.0) (2.0) F G B F G B P to market purge: F (0.010 mol/min) G (0.010 mol/min) B
Process B: Overall Mass Balance Reactions: F + G P (90% conversion) I + G B (100% conversion) G (3.0) (2.0) (0.010) B F G (0.010) H2O (1000) F (1.0) I (2.0) I does not leave the process. Bout = 2.0 mol/min P (0.99) Pout = Fin Fout H2O Pout = 1 0.01 Pout = 0.99 mol/min
H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) Process B Pout=0.9*Fin 0.99=0.9*Fin Fin= 1.11 G (3.0 mol/min) water steam liquid-gas separator 110 C F I G B F G B P P (0.11) (0.11) (1.11) (0.99) (2.0) (3.11) H2O F I (2.0) reactor F + G P I + G B 20 C (22) (1.0) liquid-gas separator 60 C (20) (0.99) F I (1.0) (2.0) (0.10) (0.10) F G B F G B (0.11) (0.11) (x) (20) (22) Bpurge Fpurge =Brecycle Frecycle P to market purge: F (0.010 mol/min) G (0.010 mol/min) B (2.0) (0.99) 2.0mol /min 0.01mol /min= x 0.1mol /min x = 20mol /min
Effect of Recycle Adjusting Recycle Ratio 100000 B in Recycle (relative reactor size) (log scale) 10000 1000 100 10 1 0.8 0.82 0.84 0.86 0.88 0.9 0.92 0.94 0.96 0.98 1 P to market (1-f purged)
Process A H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) Unit Capacity (mol/min) G (3.0 mol/min) water steam 6.2 mol/min Capacity liquid-gas separator 110 C F G B P P F I G Reactor 6.2 H2O F I reactor F + G P I + G B 20 C liquid-gas separator 60 C 3.2 mol/min Capacity Separator at 60 3.2 F I P to market (1 mol/min) F G B Separator at 80 2.2 F G B F G B B liquid-gas separator 80 C to market (2 mol/min) F G purge 2.2 mol/min Capacity Process B H2O (1000. mol/min) F (1.0 mol/min) I (2.0 mol/min) G (3.0 mol/min) water (23 mol/min) Capacity steam (26 mol/min) Capacity liquid-gas separator 110 C F I G B F G B P P H2O F I reactor F + G P I + G B 20 C Unit Capacity (mol/min) liquid-gas separator 60 C F I F G B F G B Reactor 26 Separator at 60 23 P to market purge: F (0.010 mol/min) G (0.010 mol/min) B (2.0 mol/min) (0.99 mol/min)
And the winner is Process A! (with the caveat that economic analysis is still necessary!)
Key Takeaways How to judge a design Flow rates of products to market Reactor/separator capacities Economic analysis (when available) Calculating flow rates Trace provided flow rates through the process Review overall mass balances and reaction chemistry to determine inner flow rates Use the splitter-composition rule when applicable
Setting Recycle Loop Variables Set the reactor effluent as your variables to make the math easier!
Calculation Session 4 Exercise 2.34 revisited process analysis with informal mass balances. Calculate approximate flow rates in mol/min. Exercise 3.116 process design with approximate flow rates. Assess your process: Does your process agree with an overall mass balance? Given Ain= 90 (A + P + Q)out= ? Given Bin= 10 (B + Z)out= ? What is the theoretical maximum pure P output? What is the actual maximum pure P output? Exercise 3.114 process analysis. Homework questions? Ask!
