Protective Techniques for Carboxylic Acids and Their Cleavage Methods
Learn about the importance of protecting carboxylic acids for various reasons such as preventing interference with base-catalyzed reactions and enhancing handling characteristics. Discover different protective groups like esters, 9-fluorenylmethyl esters, methoxymethyl esters, and trimethylsilyl ethoxymethyl esters, along with their formation and cleavage methods. Explore images and detailed explanations of each protective group in this informative guide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
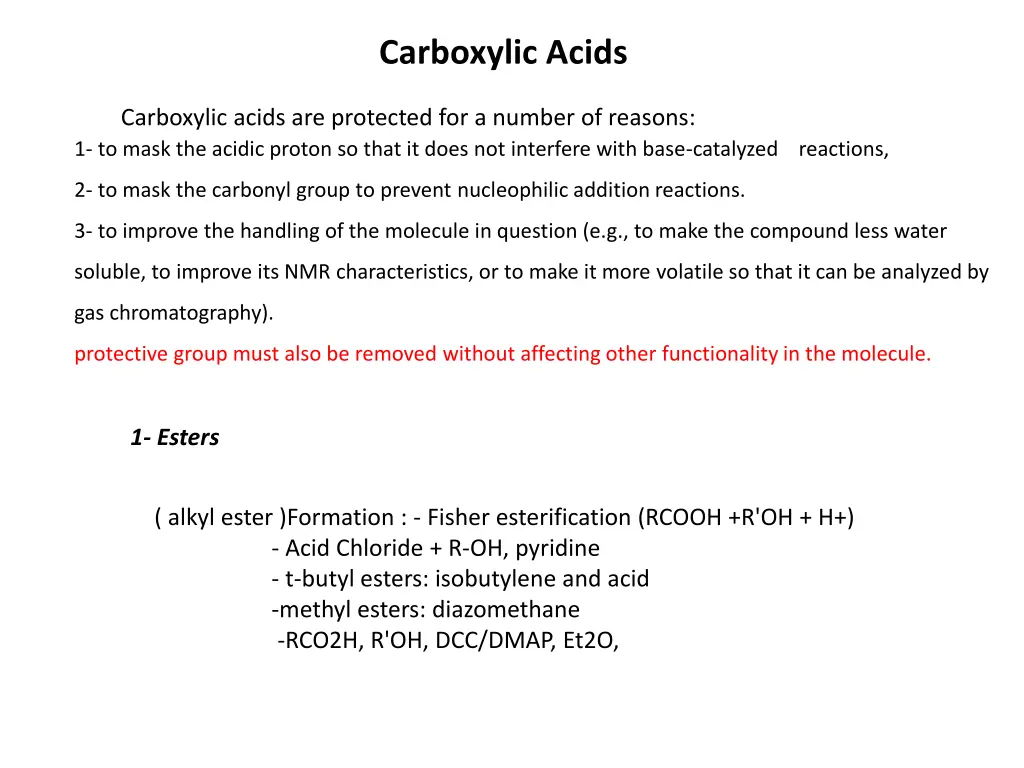
Carboxylic Acids Carboxylic acids are protected for a number of reasons: 1- to mask the acidic proton so that it does not interfere with base-catalyzed reactions, 2- to mask the carbonyl group to prevent nucleophilic addition reactions. 3- to improve the handling of the molecule in question (e.g., to make the compound less water soluble, to improve its NMR characteristics, or to make it more volatile so that it can be analyzed by gas chromatography). protective group must also be removed without affecting other functionality in the molecule. 1- Esters ( alkyl ester )Formation : - Fisher esterification (RCOOH +R'OH + H+) - Acid Chloride + R-OH, pyridine - t-butyl esters: isobutylene and acid -methyl esters: diazomethane -RCO2H, R'OH, DCC/DMAP, Et2O,
Cleavage: - LiOH, THF, H2O - enzymatic hydrolysis - t-butyl esters are cleaved with aqueous acid
9-Fluorenylmethyl Esters (Fm) 9-Fluorenylmethyl esters of N-protected amino acids were prepared using the DCC/DMAP imidazole-catalyzed transesterification of protected amino acid active esters with FmOH. Cleavage is accomplished either with diethylamine or piperidine in CH2C12 at rt for 2 h. No racemization was observed during formation or cleavage of the Fm esters. The Fm ester is cleaved slowly by hydrogenolysis, but complete selectivity for hydrogenolysis of the benzyloxycarbonyl group could not be obtained. Fm esters also improved the solubility of protected peptides in organic solvents. cleaved with mild base (Et2NH, piperidine) Methoxymethyl (MOM) Ester: RCOOCH2OCH3 1. CH3OCH2C1, Et3N, DMF, 25 , 1 h.1 2. CH3OCH2OCH3, Zn/BrCH2CO2Et, 0 ; CH3COC1, 0-20 , 2 h, 75-85%.2 A number of methoxymethyl esters were prepared by this method, which avoids the use of the carcinogen chloromethyl methyl ether.
Trimethylsilyl)ethoxymethyl Ester (SEM) The SEM ester was used to protect a carboxyl group where DCC-mediated esterification caused destruction of the substrate. It was formed from the acid and SEM chloride SEM groups are cleaved by treatment with fluoride ion. Trimethylsilyl)ethyl Esters
