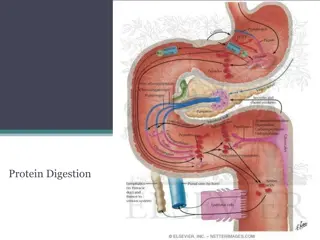
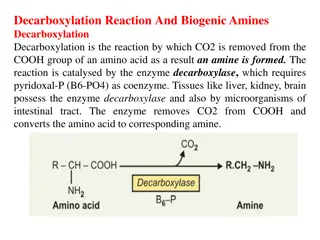
Proteins and Amino Acids in Biochemistry
Dive into the world of proteins and amino acids with Ms. P. H. Giri from the Department of Microbiology at Deogiri College, Aurangabad. Learn about the essential and non-essential amino acids, their functions, and importance in maintaining health. Explore the structural components and nutritional aspects of proteins in living organisms.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Topic Proteins-Amino Acids Presented by Ms. P. H. Giri Department of Microbiology Deogiri College, Aurangabad
B.SC. F. Y. SEMESTER II PAPER NO. V BASIC BIOCHEMISTRY UNIT 3 PROTEINS Ms. PriyankaH. Giri
INTRODUCTION: The term protein is derived from the Greek word - Proteios meaning 'primary' or 'holding first place.' Proteins are the chief constituents of all living matter. They contain -C, H, N, and S and sometimes phosphorus also. Proteins may be defined as, 'the high molecular weight mixed polymers of -amino acids, joined together with peptide (-CONH ) linkage. It may also be defined as, 'the complex nitrogenous substances which are found in the all biological systems.
AMINO ACIDS: Amino acids are the compounds containing one or more amino groups and one or more carboxyl group in the same molecule.' They are usually classified into , , - etc., according to the relative positions of the two functional groups. Generally, amino acids can be represented by the structure -
From the nutritional point of view amino acids are of two types: (1) Essential or indispensable amino acids (2) Non-essential or dispensable amino acids
Essential or indespensable amino acids: Essential amino acids are those which cannot be synthesized by the animal organisms from substances ordinarily present in the diet, at a speed commensurate with normal growth and hence are always supplied to organism in the form of dietary proteins. There are eight essential amino acids which have special importance to human beings. These are - (1) Valine (4) Phenylalanine (7) Tryptophan (2) Leucine (5) Threonine (6) Methionine (8) Lysine (3) Isoleucine
Adequate amount of essential amino acids are required to maintain the proper nitrogen balance, otherwise the deficiency of which may cause - nervous breakdown, inhibition of full mental growth, and even may cause death in young ones with complete deficiency.
Non-essential or dispensable amino acids : Non-essential amino acids are those which can be synthesized by the body and hence are not requisite components of the diet. These amino acids are derived from the carbon skeleton's of lipids and carbohydrate metabolism or from the transformation of essential amino acids. Non-essential amino acids may be synthesized in individual cells from simple starting materials containing -C, H, O, S and N. In human beings there are Twelve non-essential amino acids which have special significance for the human beings. These are - (1) Alanine (2) Argenine 3) Aspartic acid (4) Aspargine (5) Tyrosine (6) Cysteine(7) Glutamicacid (8) Glutamine (9) Glycine(10) Histidine (11) Proline (12) Serine
CLASSIFICATION OF AMINO ACIDS: Depending on their reaction in solution, amino acids can be classified into three groups. (I) Neutral (II) Acidic (III) Basic
PHYSICAL AND CHEMICAL PROPERTIES OF AMINO ACIDS: (1) Solubility:All amino acids are soluble in water. (2) Optical activity:All standard amino acids except glycinehave an asymmetric carbon atom. Due to this amino acids are optically active. (3) Acid - Base Behaviour: Amino acids contain both the acidic and basic groups which are ionizable. The amino acids behave as 'amphoteric electrolytes and give both anions and cations in solution, i.e. Zwitterions, in which proton from the carboxyl group has been transferred to the amino group and thus a dipolar (Zwitterion) ion contains both a positive and a negative charge.
An electrolytic behaviour of the amino acids in different media: In an acidic solution, an amino acid behaves as a protonated derivative and hence migragesto the cathode; while in alkaline solution the same amino acid behaves as an anion derivative and hence migrates to the anode.
With the addition of proton to an amino acid converts it into cation, while the addition of base converts it into an anion, hence it is expected that at a definite pH value, the acidic and basic properties of the amino acid must be balanced and hence electrically neutral. This pH at which it gives off equal no. of anions and cationsand does not migrate when subjected to electric field is referred as the 'isoelectric pH' of that amino acid. And the point of the pH, at which there is no net charge on the amino acid molecule and which does not migrate to any electrode under the influence of electric current is known as 'isoelectric point'.