Proton Nuclear Magnetic Resonance Spectroscopy Overview
Dive into the world of Proton Nuclear Magnetic Resonance (PMR or 1H NMR) Spectroscopy with insights on its definition, applications, historical significance, and available techniques for structure elucidation. Discover the basic components, electromagnetic radiation, and wavelength concepts associated with this powerful analytical technique. Learn from Dr. Satish U. Deshmukh at Deogiri College, Aurangabad, about the fundamental principles and advancements in NMR spectroscopy.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
Proton Nuclear Magnetic Resonance Spectroscopy Dr. Satish U. Deshmukh Department of Chemistry Deogiri College Aurangabad 1
Proton Nuclear Magnetic Resonance (PMR OR lH NMR) Spectroscopy Definition :- The study of interaction between electromagnetic radiation (EMR ) and unknown sample or matter or compounds Except mass Spectroscopy : Its requires high electron beam Nuclear magnetic resonance spectroscopy (NMR) is a commonly used and most powerful analytical technique used Characterized organic molecules The source of energy in Nuclear magnetic resonance spectroscopy (NMR) is radio waves which have long wavelengths, and thus low energy and frequency. 2
Proton Nuclear Magnetic Resonance Spectroscopy NMR spectroscopy deals with nuclear magnetic transitions between magnetic energy levels of the nuclei in molecules. First observed in 1945 separately Independently studied done by both Purcell at Harvard University and Bloch at Stanford University Finally to use of NMR to the investigate of molecules was applying first compound studied in year of 1951 and molecules was CH3CH2OH In year 1952 Purcell and Bloch awarded the Nobel Prize in Physics for their discovery. NMR spectroscopy is most powerful tools to use for elucidation of organic compounds and inorganic Chemistry 3
Proton Nuclear Magnetic Resonance Spectroscopy Techniques available for Structure Elucidation Chemical Method Involves Chem. change in a sample to determine unknown sample Large amount Time consuming process Sample can not recover Tentative structure Spectroscopy method Without Involves Chem. Change in sample to unknown sample 1) to Very less amount of sample Very short time Sample can be recover Absolutes Structure 2 3 4 5 4
Proton Nuclear Magnetic Resonance Spectroscopy Basic Component in Spectroscopy Electromagn etic radiation Detector Matter 5
Proton Nuclear Magnetic Resonance Spectroscopy Electromagnetic radiation :- It is two phases electric and magnetic EMR associated with electric field and magnetic field perpendicular to each other Wavelength ( ) :- Definition: Wavelength can be defined as the distance between two successive crests or troughs of a wave. It is measured in the direction of the wave Meter , M Centimetres, Nano-meter Millimicron 1 = 1 10-10 m 1 10-8 m 6
Proton Nuclear Magnetic Resonance Spectroscopy Wave Number :- Definition: Wave number can be defined as reciprocal wavelength = 1/ ( ) Meter, Centimetres, Millimicron Frequency :- Definition: Frequency can be defined in a given time no. of waves or photons passing though the particular point in spaces 7
Proton Nuclear Magnetic Resonance Spectroscopy Amplitude (A):- Definition: distance between a peak or a valley and the equilibrium point amplitude is the vertical Depends upon maxima or minima we called High or low Energy E= h E= h. C/ E = h. C. 1/ 8
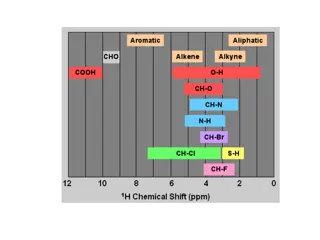
Types of Spectroscopy Ultraviolet-visible spectroscopy = Conjugation Infrared (IR) and Near Infrared = Functional Group 1H Nuclear magnetic resonance spectroscopy (NMR) = Proton 13C Nuclear magnetic resonance spectroscopy (NMR) = Carbon 19F Nuclear magnetic resonance spectroscopy (NMR) = Fluorine 31P Nuclear magnetic resonance spectroscopy (NMR) = Phosphorous Mass spectrometry = Molecular Wt. ESR spectroscopy = Free Radical Microwave spectroscopy = Bond Length Mossbauer spectroscopy = Oxidation State Photoelectron spectroscopy = Relative Energies of Electrons in atoms and molecules X-Ray = Absolutes Structure 9
Proton Nuclear Magnetic Resonance Spectroscopy Nuclear Magnetic Resonance active Nuclei There are some investigational rules which determine the net spin of a nucleus given as below. 1 ) If the number of neutrons and the number of protons are both even, then the nucleus has NO spin (I = 0). Example: For 12C, 16O, 32S = NMR inactive 2) If the number of neutrons and the number of protons are both odd, then the nucleus has an integer spin (I = 1, 2, 3 and so on). Example: 2H, 10B, 14N are NMR active. 3) If the number of neutrons plus the number of protons is odd (odd mass number or we can say odd number of neutrons/protons and even number of protons/neutrons), Then the nucleus has a half-integer spin (I = n/2, where n is an odd integer; I = 1/2, 3/2, 5/2 and so on) Example: 1H, 13C, 15N, 31P are NMR active. 10
Proton Nuclear Magnetic Resonance Spectroscopy THEORY Moving charged particle generates a magnetic field. All the atomic nuclei or molecules positively are charged particles and as a result molecules or spinning nuclei or spin active nuclei. All spin active nuclei has been generate a magnetic field along the axis of rotation. We can understands that the spinning nuclei behave like a small magnet with a magnetic ( ). In the absence of external magnetic field all atomic nuclei or molecules positively are charged particles when we applied an external magnetic field. 11
Proton Nuclear Magnetic Resonance Spectroscopy 12
Proton Nuclear Magnetic Resonance Spectroscopy THEORY All nuclei atomic nuclei or molecules positively are charged particles align themselves either with or against the field of the external magnet. Quantum mechanics explains when a magnetic active nuclei with nuclear spin (I) is placed in a uniform magnetic field, its magnetic dipole or magnetic moment has been align in only this formula ( 2I+1 ). All atomic nuclei or molecules positively are charged particles with Spin I = . Nuclei two possible orientations 1) Upward direction 2) Downwards direction 13
Proton Nuclear Magnetic Resonance Spectroscopy In the presence of an external magnetic field (H0) or applied magnetic fields some of the nuclei organize themselves in the such a approach of the external magnetic field while others in the opposite direction. More energy is required for a nucleus to align against the external magnetic field than to align it with the external magnetic field. Therefore, nuclei which align with the field are in the 1) Lower energy state called -spin state and those which align against the field . 2) Higher energy state called as -spin state. 14
Proton Nuclear Magnetic Resonance Spectroscopy I= -1/2, Higher energy The radiation less transitions by which a nucleus in an upper spin state returns to a lower spin state are called relaxation processes. ~E = hv I = +1/2, Lower energy, a-spin state Orientation of nuclear magnetic dipoles in an external magnetic field Ho. 15
Proton Nuclear Magnetic Resonance Spectroscopy Thank you 16