Qualitative Tests of Proteins & Amino Acids for Identification
Conduct qualitative tests to identify amino acids and proteins based on specific characteristics like side chains and functional groups. Explore assays such as Ninhydrin, Xanthoproteic, Lead Sulfite, and Millon's test. Learn about the unique reactions that help in detection, including the formation of brick-red precipitates and lead sulfide.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
LAB 6 : QUALITATIVE TESTS OF PROTEINS & AMINO ACIDS DR. SHAIMAA MUNTHER
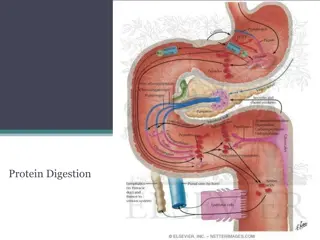
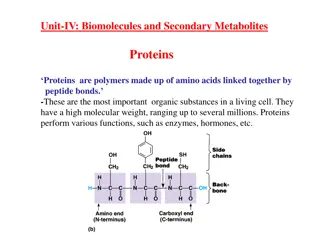
Amino acids & Protein Analysis A. Solubility test. B. Identification tests for amino acids : There number of test to detect the presence of amino acid ,These are largely depend on the nature of amino acids side chain usually. Example of these tests are : Ninhydrin test: for -L amino acids 1. Xanthoproteic test: for Aromatic amino acids 2. Lead sulfite test: detection of amino acids containing sulfhydryl group (-SH) 3. Millon's test: for amino acids containing hydroxy phenyl group 4. C. Identification tests for proteins : The presence of proteins in a solution is often detected by general tests, such as biuret or specific tests that depend on the presence of a specific proteins.
Millons Test Objective: This test is specific for tyrosine. Because it is the only amino acid containing a phenol group. Note: phenol group, a hydroxyl group attached to benzene ring. Millon s reagent contains mercury and HNO3 and is very toxic, corrosive a strong oxidant, an irritant, and can cause burns
Principle: The phenol group of tyrosine is first nitrated by nitric acid in the test solution. Then the nitrated tyrosine complexes mercury ions in the solution to form a brick-red , appearance of red color is positive test. Note: all phenols (compound having benzene ring and OH attached to it) give positive results in Millon s test.
Procedure To 2 ml of protein solution in a test tube, add 3 drops of Millon s reagent. Mix well and heat directly on a small flame for 5 min . A white ppt is formed with albumin and casein (but not gelatin); the ppt gradually turns into brick red.
Lead Sulfite Test Objective: This test specific for SH [sulfhydryl group] containing amino acid (Cysteine). Principle: - Sulfur in cystine, is converted to sodium sulfide by boiling with 10% NaOH. - The Na2S can be detected by the precipitation of PbS (lead sulfide) from an alkaline solution, when adding lead acetate Pb (CH3COO)2.
Procedure 1. Place 1 ml of 2% casein, 2% egg albumin, 2% peptone, 2% gelatin and 0.1 M cysteine into separate, labeled test tubes. 2. Add 2 ml of 10 % aqueous sodium hydroxide. Add 5 drops of 10 % lead acetate solution. 3. Stopper the tubes and shake them. Remove the stoppers and heat in a boiling water bath for 5 minutes. Cool and record the results.
Biuret Test Biuret test is specific for protein and polypeptide Biuret structure: it is result of condensation of two molecule of urea Principle: The biuret reagent (copper sulfate in a strong base) reacts with peptide bonds in proteins to form a blue to violet chelate complex known as the biuret complex . This color change the number of peptide bonds in the solution, so the more protein, the more intense the change. is dependent on
A chelate is a chemical compound composed of a metal ion and a chelating agent. A chelating agent is a substance whose molecules can form several bonds to a single metal ion. In other words, a chelating agent is a multidentate ligand.
Procedure To 0.5 ml of protein solution in a test tube, add 100 l of reagent incubation 5 min Result : Blue to violet coloured complex indicate +ve result
Observations Interpretation No change ( solution remains blue ) Proteins are not present The solution turns from blue to violet( purple) Proteins are present Peptides are present ( Peptides or peptones are short chains of amino acid residues) The solution turns from blue to pink
Albumin Test Albumin is one of the most important carrier proteins in plasma and binds hormones, vitamins, ions and drugs for transportation throughout the body. Albumin is the most abundant plasma protein and keeps fluid from leaking out of blood vessels, maintaining osmotic pressure. Measurement of albumin is useful in the diagnosis of liver diseases such as cirrhosis; because albumin is produced in the liver the level of albumin in the blood drops when the liver is damaged. Decreased albumin levels can also occur in nephrotic syndrome, severe inflammation, or shock. The blood albumin concentration increases when a person in dehydrated. Albumin measurement can assist with monitoring nutrition and general health status.
Albumin Test This direct colorimetric method utilises bromocresol green (BCG) dye. At pH 3.8 albumin in the sample reacts with bromocresol green to form a green coloured complex. The colour intensity is proportional to the concentration of albumin.
Procedure To 0.5 ml of protein solution in a test tube, add 100 l of reagent incubation 5 min Result : green coloured complex indicate +ve result