Quantum Numbers and Spherical Harmonics in Hydrogen Atom
Understand the significance of quantum numbers (n, l, m) and their impact on the 3D behavior of wavefunctions in hydrogen atoms. Explore the interplay of orbital and magnetic numbers, nodal surfaces, and spherical harmonics in polar plots. Dive into the radial and angular parts of the hydrogen atom wavefunction for in-depth insights.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
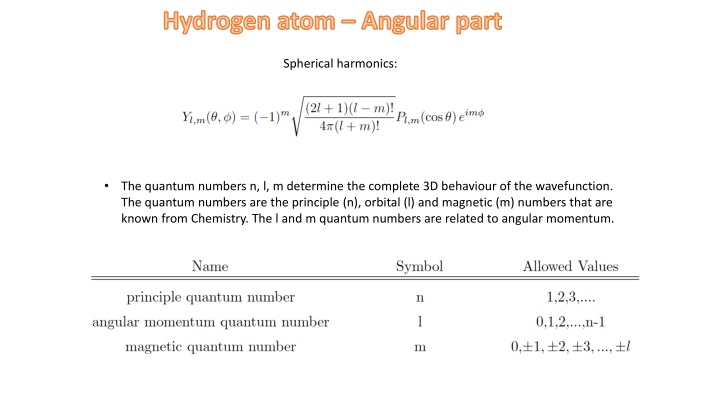
Hydrogen atom Angular part Spherical harmonics: The quantum numbers n, l, m determine the complete 3D behaviour of the wavefunction. The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum.
s-type orbital Spherical harmonics normal plots l=0 p-type orbital l=1 d-type orbital l=2 f-type orbital l=3 l=4 m=0 m=1 m=2 m=3 m=4
The probability densities in r and ? have zeros for several values. These result in nodal surfaces where the probability of finding the electron vanishes. This is because bound particles are standing waves! All eigenstates (except for the ground state) are degenerate in energy since the energy only depends on n. Now let s investigate the spherical harmonics using polar plots. In these plots, the distance from origin to curve in direction ? is given by Yl,m(?,?). 3D dependence from rotating around z-axis (ie, through all ?).
Spherical harmonics polar plots l=0, m=0 l=1, m=-1,0,1 l=2, m=-2,-1,0,1,2 l=3, m=-3,-2,-1,0,1,2,3
The Hydrogen atom wavefunction: Born s rule: Probability of finding the particle within some volume: