Reaction Mechanisms
Reaction mechanisms involve the step-by-step pathways of molecular events within a chemical reaction, elucidating how reactants form products. Elementary reactions play a crucial role in determining the overall outcome of a reaction. Molecularity categorizes reactions based on the number of molecules needed for collision, ranging from unimolecular to termolecular. Rate equations for elementary reactions and bimolecular reactions provide insights into the dependence on reactant concentrations for reaction rates.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
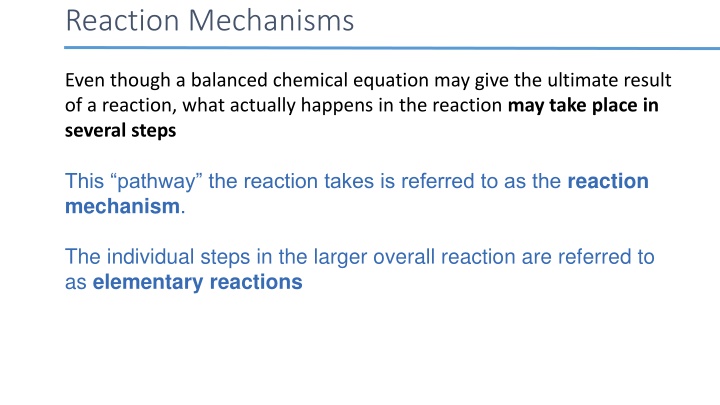
Reaction Mechanisms Even though a balanced chemical equation may give the ultimate result of a reaction, what actually happens in the reaction may take place in several steps This pathway the reaction takes is referred to as the reaction mechanism. The individual steps in the larger overall reaction are referred to as elementary reactions
Reaction Mechanisms Consider the reaction of nitrogen dioxide with carbon monoxide. ) g ( CO ) g ( NO 2 + + + + NO ) g ( CO ) g ( 2 This reaction is believed to take place in two steps. + + + + NO ) g ( 2 NO ) g ( 2 NO ) g ( 3 NO ) g ( (elementary reaction) + + + + NO ) g ( 3 CO ) g ( NO ) g ( 2 CO ) g ( 2 (elementary reaction)
Reaction Mechanisms Each step is a singular molecular event resulting in the formation of products. Note that NO3 does not appear in the overall equation, but is formed as a temporary reaction intermediate. + + + + NO ) g ( 2 NO ) g ( 2 NO ) g ( 3 NO ) g ( (elementary reaction) + + + + NO ) g ( 3 CO ) g ( NO ) g ( 2 CO ) g ( 2 (elementary reaction)
Reaction Mechanisms Each step is a singular molecular event resulting in the formation of products. The overall chemical equation is obtained by adding the two steps together and canceling any species common to both sides. + + + + NO ) g ( 2 NO ) g ( 2 NO ) g ( 3 NO ) g ( + + + + NO ) g ( 3 CO ) g ( NO ) g ( 2 CO ) g ( 2 + + + + + + NO ) g ( 2 NO ) g ( 2 NO ) g ( 3 CO ) g ( + + + + + + NO ) g ( 3 NO ) g ( NO ) g ( 2 CO ) g ( 2
Molecularity We can classify reactions according to their molecularity, that is, the number of molecules that must collide for the elementary reaction to occur. A unimolecular reaction involves only one reactant molecule. A bimolecular reaction involves the collision of two reactant molecules. A termolecular reaction requires the collision of three reactant molecules. Higher molecularities are rare because of the small statistical probability that four or more molecules would all collide at the same instant.
Rate Equations for Elementary Reactions Since a chemical reaction may occur in several steps, there is no easily stated relationship between its overall reaction and its rate law. products A For example, consider the generic equation below. The rate is dependent only on the concentration of A; that is, Rate= = k[A]
Rate Equations for Bimolecular Elementary Reactions However, for the reaction B + + products A the rate is dependent on the concentrations of both A and B. Rate= = k[A][B]
Rate Equations for Termolecular Elementary Reactions For a termolecular reaction B + + + + products A C the rate is dependent on the populations of all three participants. Rate= = k[A][B][C]
Rate Equations for Termolecular Elementary Reactions Note that if two molecules of a given reactant are required, it appears twice in the rate law. For example, the reaction B + + products 2A would have the rate law: 2 Rate= = k[A] = = k[A][A][B] Rate [B] or
Rate Equations for Elementary Reactions So for an elementary reaction,the coefficient of each reactant becomes the power to which it is raised in the rate law for that reaction. Many chemical reactions occur in multiple steps and it is, therefore, impossible to predict the rate law based solely on the overall reaction.
Rate Laws and Mechanisms Consider the reaction NO 2 + + (g) F (g) 2 NO F(g) 2 2 2 Experiments performed with this reaction show that the rate law is = = Rate k[NO ][F ] 2 2 This implies that the reaction above is not an elementary reaction but rather the result of multiple steps.
Rate Determining Step In multiple-step reactions, one of the elementary reactions in the sequence is often slower than the rest. The overall reaction cannot proceed any faster than this slowest rate-determining step.
Rate Determining Step In multiple-step reactions, one of the elementary reactions in the sequence is often slower than the rest. Our previous example occurs in two elementary steps where the first step is much slower. k + + NO F(g) + + (slow) NO (g) F (g) F(g) 1 2 2 2 k F(g) + + (fast) NO (g) NO F(g) 2 2 2 + + NO 2 (g) F (g) 2 NO F(g) 2 2 2
Rate Determining Step In multiple-step reactions, one of the elementary reactions in the sequence is often slower than the rest. Since the overall rate of this reaction is determined by the slow step, it seems logical that the observed rate law isRate = k1[NO2][F2]. k + + NO F(g) + + NO (g) F (g) F(g) 1 2 2 2
Rate Determining Step In a mechanism where the first elementary step is the rate-determining step, the overall rate law is simply expressed as the elementary rate law for that slow step. A more complicated scenario occurs when the rate- determining step contains a reaction intermediate, as you ll see in the next section
Rate Determining Step Mechanisms with an Initial Fast Step There are cases where the rate- determining step of a mechanism contains a reaction intermediate that does not appear in the overall reaction. The experimental rate law, however, can be expressed only in terms of substances that appear in the overall reaction.
Rate Determining Step Consider the reduction of nitric oxide with H2. ) g ( H 2 ) g ( NO 2 2 + + A proposed mechanism is: + + N ) g ( 2 2 H ) g ( O 2 k1 2 NO k-1 N 2O (fast, equilibrium) 2 k + + + + N O H N O H O (slow) 2 2 2 2 2 2 k + + + + N O H N H O (fast) 3 2 2 2 2 It has been experimentally determined that the rate law is Rate = k [NO]2[H2]
Rate Determining Step The rate-determining step (step 2 in this case) generally outlines the rate law for the overall reaction. = = Rate k [ N O ][ H ] 2 2 2 2 (Rate law for the rate-determining step) As mentioned earlier, the overall rate law can be expressed only in terms of substances represented in the overall reaction and cannot contain reaction intermediates such as N2O2. We can do this by looking at the first step, which is fast and establishes equilibrium
Rate Determining Step The rate-determining step (step 2 in this case) generally outlines the rate law for the overall reaction. ][ O N [ k Rate 2 2 2 = = H ] 2 At equilibrium, the forward rate and the reverse rate are equal. k ] NO [ k 1 = = Therefore, k ( ] O N [ = = And 2 [ N O ] 1 2 2 2 / k )[ NO ] 2 2 1 1 k k 2 = = 2 1 Rate [ NO ] [ H ] 2 k 1
Rate Determining Step k k 2 = = 2 1 Rate [ NO ] [ H ] 2 k 1 If we replace the constants (k2k1/k-1) with and effective k, we obtain the observed rate law:Rate = k[NO]2[H2].
Rate Equations for Elementary Reactions Mechanisms with an Initial Fast Step There are cases where the rate- determining step of a mechanism contains a reaction intermediate that does not appear in the overall reaction. The experimental rate law, however, can be expressed only in terms of substances that appear in the overall reaction.
Rate Equations for Elementary Reactions Mechanisms with an Initial Fast Step There are cases where the rate- determining step of a mechanism contains a reaction intermediate that does not appear in the overall reaction. The experimental rate law, however, can be expressed only in terms of substances that appear in the overall reaction.
Reaction Mechanisms Often we find that seemingly simple, first order reactions are really the result of a complex series. Among these are unimolecular isomerization Another type of unimolecular dynamics involves the decomposition of a molecule, for example: C3H8 C3H7 + H N2O5 NO2+ NO3. But we know that these are stable molecules, how do they isomerize or dissociate? The answer was first hinted at by Lindemann.
Lindemann Mechanism Lindemann thought that collisions could activate a molecule to a state which could either fall apart or following another collision, return to the ground state. He modeled the unimolecular reaction of molecules A in a bath (a lot) of M A + M A* + M (-1) A* + M A + M A* Products If only a small number of the activated molecules fall apart then the rates of reaction (1) and (-1) are equal k1 [A][M] = k-1 [A*][M] And a simple rearrangement yields (1) k1 k-1 k2 (2) * * k [ ][ ] [ ] A M A = = 1 A A k M 1
Unimolecular Reactions Thus the concentration of activate molecules ?1 ? 1 ? = ? The rate at which products appear ?? ??= ?2 ?1 ? 1 ? So unimolecular reactions are first order in [A]
Chain Reactions Initiation: Propagation: Cl2 2Cl Cl + CH4 CH3 + HCl CH3 + Cl2 CH3Cl + Cl CH3 + Cl CH3Cl CH3 + CH3 H3CCH3 Cl + Cl Cl2 Termination:
Catalysts A catalystis a substance that provides a good environment for a reaction to occur, thereby increasing the reaction rate without being consumed by the reaction. To avoid being consumed, the catalyst must participate in at least one step of the reaction and then be regenerated in a later step.
Catalysts A catalystis a substance that provides a good environment for a reaction to occur, thereby increasing the reaction rate without being consumed by the reaction. Its presence increases the rate of reaction by either increasing the frequency factor, A (from the Arrhenius equation) or lowering the activation energy, Ea.
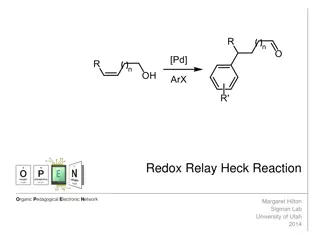
Homogeneous Catalysts Homogeneous catalysis is the use of a catalyst in the same phase as the reacting species. The oxidation of sulfur dioxide using nitric oxide as a catalyst is an example where all species are in the gas phase. NO ( g ) + + SO 2 ) g ( 2 O ) g ( 2 SO 2 ) g ( 3
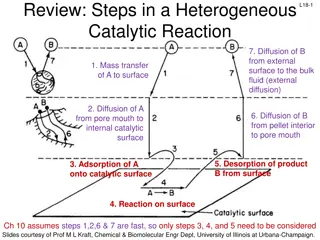
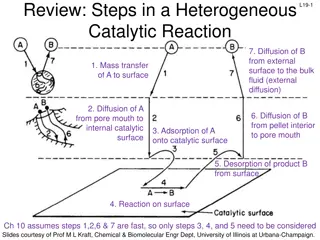
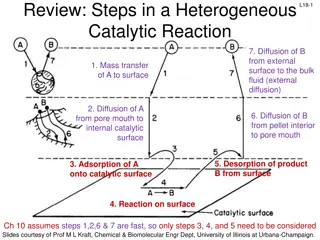
Heterogeneous Catalysts Heterogeneous catalysis is the use of a catalyst that exists in a different phase from the reacting species, usually a solid catalyst in contact with a liquid or gaseous solution of reactants. Such surface catalysis is thought to occur by chemical adsorption of the reactants onto the surface of the catalyst.
Enzyme Catalysis Enzymes have enormous catalytic activity. The substance whose reaction the enzyme catalyzes is called the substrate
Enzyme Catalysis Reduction in activation energy resulting from the formation of an enzyme-substrate complex. Formation of the ES complex is exothermic (A). Formation of the activated complex ES* from it requires energy (D) which is less than the activation energy needed for the direct reaction (C) by an amount (B)
Unimolecular Reactions Thus the concentration of activate molecules ?1 ? 1 ? = ? The rate at which products appear ?? ??= ?2 ?1 ? 1 ? So unimolecular reactions are first order in [A]
Unimolecular Reactions Thus the concentration of activate molecules ?1 ? 1 ? = ? The rate at which products appear ?? ??= ?2 ?1 ? 1 ? So unimolecular reactions are first order in [A]
Unimolecular Reactions Thus the concentration of activate molecules ?1 ? 1 ? = ? The rate at which products appear ?? ??= ?2 ?1 ? 1 ? So unimolecular reactions are first order in [A]
Unimolecular Reactions Thus the concentration of activate molecules ?1 ? 1 ? = ? The rate at which products appear ?? ??= ?2 ?1 ? 1 ? So unimolecular reactions are first order in [A]