Reactions Between Metals and Acids: Redox Reactions
Learn about redox reactions between metals and acids, where metals donate electrons to hydrogen ions, displacing hydrogen as a gas. Understand concepts of oxidation, reduction, ionic half-equations, extraction of metals, and more. Discover how different metals react with acids and the principles of oxidation and reduction in terms of electrons.
Download Presentation

Please find below an Image/Link to download the presentation.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author. If you encounter any issues during the download, it is possible that the publisher has removed the file from their server.
You are allowed to download the files provided on this website for personal or commercial use, subject to the condition that they are used lawfully. All files are the property of their respective owners.
The content on the website is provided AS IS for your information and personal use only. It may not be sold, licensed, or shared on other websites without obtaining consent from the author.
E N D
Presentation Transcript
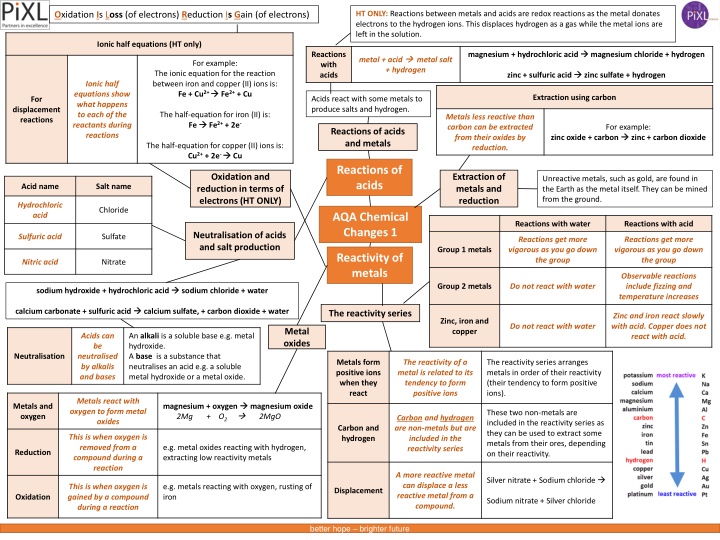
HT ONLY: Reactions between metals and acids are redox reactions as the metal donates electrons to the hydrogen ions. This displaces hydrogen as a gas while the metal ions are left in the solution. Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons) Ionic half equations (HT only) magnesium + hydrochloric acid magnesium chloride + hydrogen Reactions with acids metal + acid + hydrogen metal salt For example: The ionic equation for the reaction between iron and copper (II) ions is: Fe + Cu2+ Fe2+ + Cu zinc + sulfuric acid zinc sulfate + hydrogen Ionic half equations show what happens to each of the reactants during reactions Extraction using carbon Acids react with some metals to produce salts and hydrogen. For displacement reactions The half-equation for iron (II) is: Fe Fe2+ + 2e- Metals less reactive than carbon can be extracted from their oxides by reduction. For example: zinc + carbon dioxide Reactions of acids and metals zinc oxide + carbon The half-equation for copper (II) ions is: Cu2+ + 2e- Cu Reactions of acids Extraction of metals and reduction Oxidation and reduction in terms of electrons (HT ONLY) Unreactive metals, such as gold, are found in the Earth as the metal itself. They can be mined from the ground. Acid name Salt name Hydrochloric acid Chloride AQA Chemical Changes 1 Reactions with water Reactions with acid Neutralisation of acids and salt production Sulfuric acid Sulfate Reactions get more vigorous as you go down the group Reactions get more vigorous as you go down the group Group 1 metals Reactivity of metals Nitric acid Nitrate Observable reactions include fizzing and temperature increases Group 2 metals Do not react with water sodium hydroxide + hydrochloric acid sodium chloride + water calcium carbonate + sulfuric acid calcium sulfate, + carbon dioxide + water The reactivity series Zinc and iron react slowly with acid. Copper does not react with acid. Zinc, iron and copper Do not react with water Metal oxides Acids can be neutralised by alkalis and bases An alkali is a soluble base e.g. metal hydroxide. A base is a substance that neutralises an acid e.g. a soluble metal hydroxide or a metal oxide. Neutralisation Metals form positive ions when they react The reactivity of a metal is related to its tendency to form positive ions The reactivity series arranges metals in order of their reactivity (their tendency to form positive ions). Metals react with oxygen to form metal oxides magnesium + oxygen 2Mg + O2 2MgO magnesium oxide Metals and oxygen These two non-metals are included in the reactivity series as they can be used to extract some metals from their ores, depending on their reactivity. Carbon and hydrogen are non-metals but are included in the reactivity series Carbon and hydrogen This is when oxygen is removed from a compound during a reaction e.g. metal oxides reacting with hydrogen, extracting low reactivity metals Reduction A more reactive metal can displace a less reactive metal from a compound. Silver nitrate + Sodium chloride This is when oxygen is gained by a compound during a reaction e.g. metals reacting with oxygen, rusting of iron Displacement Oxidation Sodium nitrate + Silver chloride better hope brighter future
HT ONLY: Reactions between metals and acids are redox reactions as the metal donates electrons to the hydrogen ions. This displaces hydrogen as a gas while the metal ions are left in the solution. Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons) Ionic half equations (HT only) magnesium + hydrochloric acid magnesium chloride + hydrogen metal + acid + hydrogen metal salt For example: The ionic equation for the reaction between iron and copper (II) ions is: Fe + Cu2+ Fe2+ + Cu zinc + sulfuric acid zinc sulfate + hydrogen Ionic half equations show what happens to each of the reactants during reactions Acids react with some metals to produce salts and hydrogen. The half-equation for iron (II) is: Fe Fe2+ + 2e- Metals less reactive than carbon can be extracted from their oxides by reduction. For example: zinc + carbon dioxide Reactions of acids and metals zinc oxide + carbon The half-equation for copper (II) ions is: Cu2+ + 2e- Cu Reactions of acids Extraction of metals and reduction Oxidation and reduction in terms of electrons (HT ONLY) Unreactive metals, such as gold, are found in the Earth as the metal itself. They can be mined from the ground. Acid name Salt name Hydrochloric acid AQA Chemical Changes 1 Neutralisation of acids and salt production Sulfuric acid Reactions get more vigorous as you go down the group Reactions get more vigorous as you go down the group Group 1 metals Reactivity of metals Nitric acid Observable reactions include fizzing and temperature increases Group 2 metals Do not react with water sodium hydroxide + hydrochloric acid sodium chloride + water calcium carbonate + sulfuric acid calcium sulfate, + carbon dioxide + water The reactivity series Zinc and iron react slowly with acid. Copper does not react with acid. Zinc, iron and copper Do not react with water Metal oxides Acids can be neutralised by alkalis and bases An alkali is a soluble base e.g. metal hydroxide. A base is a substance that neutralises an acid e.g. a soluble metal hydroxide or a metal oxide. The reactivity of a metal is related to its tendency to form positive ions The reactivity series arranges metals in order of their reactivity (their tendency to form positive ions). Metals react with oxygen to form metal oxides magnesium + oxygen 2Mg + O2 2MgO magnesium oxide These two non-metals are included in the reactivity series as they can be used to extract some metals from their ores, depending on their reactivity. Carbon and hydrogen are non-metals but are included in the reactivity series This is when oxygen is removed from a compound during a reaction e.g. metal oxides reacting with hydrogen, extracting low reactivity metals A more reactive metal can displace a less reactive metal from a compound. Silver nitrate + Sodium chloride This is when oxygen is gained by a compound during a reaction e.g. metals reacting with oxygen, rusting of iron Sodium nitrate + Silver chloride better hope brighter future
HT ONLY: Reactions between metals and acids are redox reactions as the metal donates electrons to the hydrogen ions. This displaces hydrogen as a gas while the metal ions are left in the solution. Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons) Ionic half equations (HT only) magnesium + hydrochloric acid magnesium chloride + hydrogen For example: The ionic equation for the reaction between iron and copper (II) ions is: Fe + Cu2+ Fe2+ + Cu zinc + sulfuric acid zinc sulfate + hydrogen Acids react with some metals to produce salts and hydrogen. The half-equation for iron (II) is: Fe Fe2+ + 2e- For example: zinc + carbon dioxide Reactions of acids and metals zinc oxide + carbon The half-equation for copper (II) ions is: Cu2+ + 2e- Cu Reactions of acids Unreactive metals Extraction of metals and reduction Oxidation and reduction in terms of electrons (HT ONLY) Acid name Salt name AQA Chemical Changes 1 Neutralisation of acids and salt production Reactions get more vigorous as you go down the group Group 1 metals Reactivity of metals Observable reactions include fizzing and temperature increases Group 2 metals sodium hydroxide + hydrochloric acid sodium chloride + water calcium carbonate + sulfuric acid calcium sulfate, + carbon dioxide + water The reactivity series Zinc and iron react slowly with acid. Copper does not react with acid. Zinc, iron and copper Metal oxides An alkali is a soluble base e.g. metal hydroxide. A base is a substance that neutralises an acid e.g. a soluble metal hydroxide or a metal oxide. The reactivity of a metal is related to its tendency to form positive ions magnesium + oxygen 2Mg + O2 2MgO magnesium oxide Carbon and hydrogen are non-metals but are included in the reactivity series e.g. metal oxides reacting with hydrogen, extracting low reactivity metals A more reactive metal can displace a less reactive metal from a compound. e.g. metals reacting with oxygen, rusting of iron better hope brighter future
HT ONLY: Reactions between metals and acids are redox reactions as the metal donates electrons to the hydrogen ions. This displaces hydrogen as a gas while the metal ions are left in the solution. Oxidation Is Loss (of electrons) Reduction Is Gain (of electrons) Ionic half equations (HT only) For example: The ionic equation for the reaction between iron and copper (II) ions is: Fe + Cu2+ Fe2+ + Cu Acids react with some metals to produce salts and hydrogen. For example: Reactions of acids and metals Reactions of acids Unreactive metals Extraction of metals and reduction Oxidation and reduction in terms of electrons (HT ONLY) Acid name Salt name AQA Chemical Changes 1 Neutralisation of acids and salt production Group 1 metals Reactivity of metals Group 2 metals Examples: The reactivity series Zinc, iron and copper Metal oxides An alkali is a A base is a magnesium + oxygen 2Mg + O2 2MgO magnesium oxide e.g. e.g. better hope brighter future